Malignant Transformation of Craniopharyngioma after Growth Hormone Replacement Therapy
A B S T R A C T
Background: We present a rare case of malignant transformation of a craniopharyngioma in a patient who received growth hormone replacement therapy.
Case Description: A 43-year-old male came to our department with a headache and visual disturbance and was admitted. Brain magnetic resonance imaging (MRI) revealed a giant tumor in the intra- and suprasellar region compressing the optic chiasma. Using a transcranial pterional approach, the tumor was removed as much as possible, with the residual mass removed later with a trans-sphenoidal approach. The initial histopathological diagnosis was adamantinomatous craniopharyngioma. Decompression of the optic chiasma was achieved and visual function improved, though residual tumor tissue remained in the parasellar region, without regrowth seen for more than 24 years. At the age of 67 years, the patient received growth hormone replacement therapy because of a deficiency, then 2 years later the residual tumor began to grow gradually. Trans-sphenoidal surgery was again performed to remove the growing tumor and histopathology results revealed an adamantinomatous craniopharyngioma with malignant transformation. Stereotactic radiation therapy was performed for the residual mass with a total dose of 60 Gy. Presently, 1 year later the patient has a stable condition, and the tumor remains controlled, while follow-up examinations are continuing.
Conclusion: A craniopharyngioma may undergo malignant transformation with continuous exposure to administered growth hormone. Close follow-up and imaging examinations are necessary to detect a change, including tumor growth in such cases.
Keywords
Malignant transformation, craniopharyngioma, growth hormone
Introduction
A craniopharyngioma, which originates from Rathke’s pouch, a derivative of the oral ectoderm, is a benign tumor that accounts for approximately 3-3.5% of all intracranial tumors [1]. Recurrence following a total resection procedure has been reported to occur in 30% of such cases, while that after subtotal resection occurs in 50-70%, thus adjuvant radiotherapy following a subtotal procedure has been recommended [2].
Malignant transformation of a craniopharyngioma rarely occurs, and the etiology and pathogenesis of those cases are unclear, though a previous study suggested a correlation with radiotherapy [3]. There are no known reports of a case with malignant transformation associated with growth hormone (GH) replacement therapy. The prognosis of individuals with a malignantly transformed craniopharyngioma is considered to be poor, though the incidence rate is low, and there is insufficient evidence regarding available treatment modalities [4]. Herein, we present a case of craniopharyngioma with a subsequent malignant transformation that occurred 2 years after GH replacement therapy without radiotherapy.
Case Description
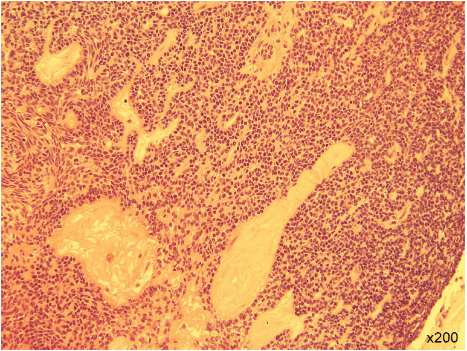
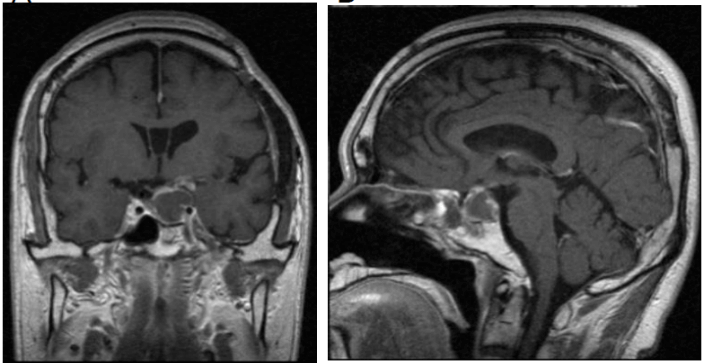
A 43-year-old male came to our department in 1992 with a headache and visual disturbance and was admitted. Brain magnetic resonance imaging (MRI) revealed a tumor in the intra- and suprasellar region compressing the optic chiasma. Using a transcranial pterional approach, tumor was removed, with the residual mass removed later with a trans-sphenoidal approach. The histopathological diagnosis was typical adamantinomatous craniopharyngioma (Figure 1). There was no cytologic atypia or mitosis. Decompression of the optic chiasma was achieved and visual function improved, though residual tumor tissue remained around the parasellar region (Figure 2). Thereafter, the residual tumor did not show regrowth for more than 24 years.
Figure 1: The histopathologic diagnosis was adamantinomatous craniopharyngioma. There were no findings of cytologic atypia or mitosis. Hematoxylin-eosin staining (original magnification x200).
Figure 2: Results of brain MRI with Gd following transcranial and trans-sphenoidal surgery. Following those procedures, residual tumor tissue remained around the parasellar region. A) Coronal section. B) Sagittal section.
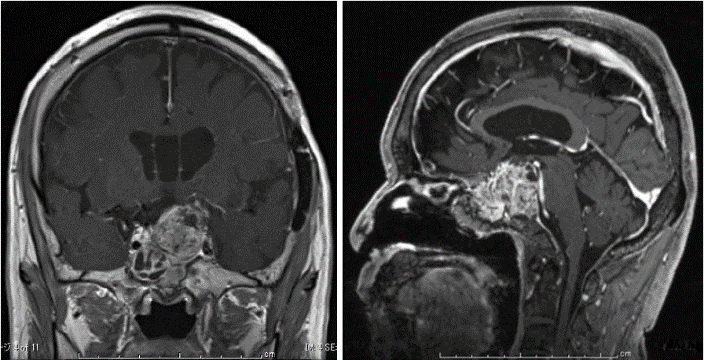
Figure 3: Results of brain MRI with Gd at 2 years after starting growth hormone therapy. The tumor showed aggressive growth. A) Coronal section. B) Sagittal section.
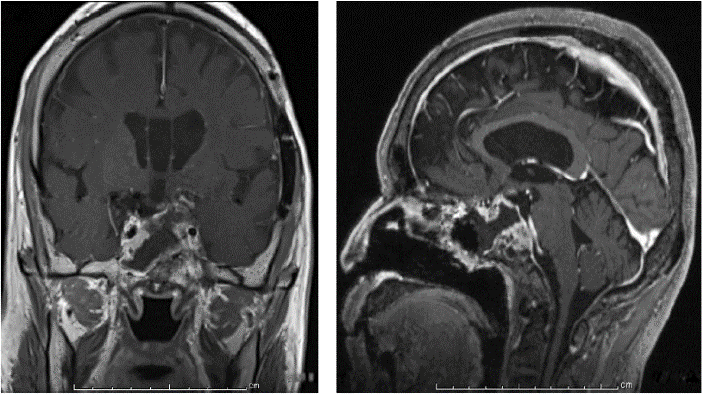
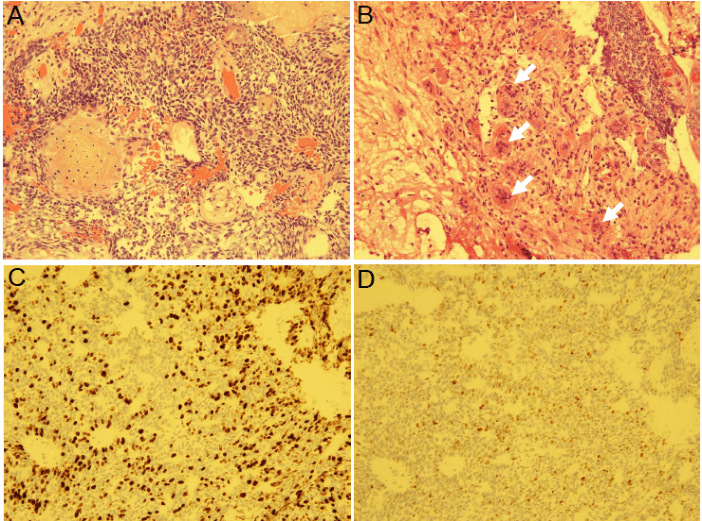
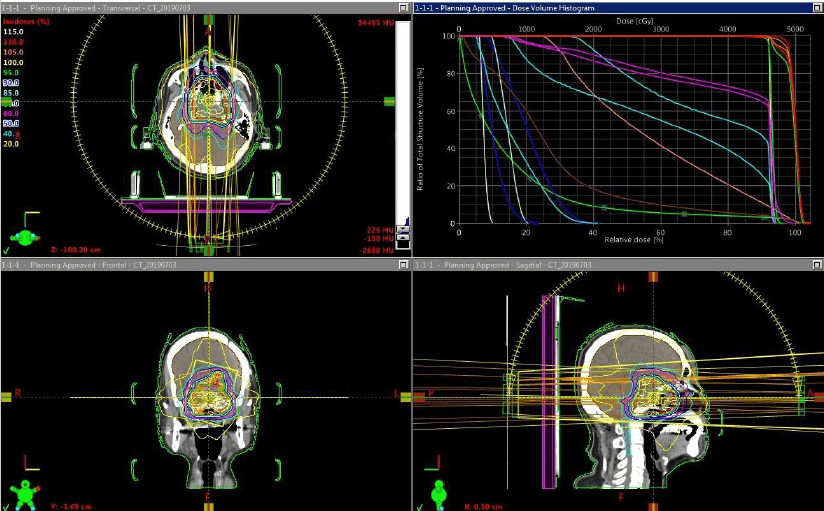
In 2016, severe GH deficiency was noted, and the patient underwent GH replacement therapy at the age of 67. However, 2 years later the residual tumor began to grow (Figure 3); thus, trans-sphenoidal surgery was again performed to remove the growing tumor (Figure 4). Histopathological results revealed an adamantinomatous craniopharyngioma with cytologically malignant features (Figure 5A). The tumor cells showed significant nuclear pleomorphism, hyperchromasia, and a high nuclear-cytoplasmic ratio, indicating malignant transformation (Figure 5B). Furthermore, immunohistochemistry findings showed a Ki67 proliferation rate >45% and overexpression of p53 (Figures 5C & 5D). To prevent growth of the residual tumor, adjuvant stereotactic radiotherapy was performed with a total dose of 60 Gy in 30 fractions (Figure 6). Presently, 1 year after radiotherapy, the patient is in stable condition, and the tumor is controlled.
Figure 4: Results of brain MRI with Gd after trans-sphenoidal surgery. A) Coronal section. B) Sagittal section.
Figure 5: Histopathology findings revealed malignant transformation of the craniopharyngioma. A) Hematoxylin-eosin stain (original magnification x200). B) Tumor cells showed significant nuclear pleomorphism (white arrows). Hematoxylin-eosin stain (original magnification x200). C) Immunohistochemistry for Ki67 (original magnification x200). D) Immunohistochemistry for p53 (original magnification x100).
Discussion
The World Health Organization (WHO) classifies a craniopharyngioma as a benign grade 1 tumor known for high recurrence and local aggressiveness despite treatment [5]. However, cases with malignant transformation are extremely rare, with the first presented in 1987, for a total of 28 reported cases, of which only 6 noted a malignant craniopharyngioma without radiotherapy [4, 6-9]. Although the exact cause and pathogenesis of such malignant transformation are unknown, previous studies have speculated a likely correlation with radiotherapy. On the other hand, in a review by Sofela AA et al., there was a poor correlation found between malignant transformation and radiotherapy [5]. The present case also showed transformation to malignancy without radiotherapy; thus, we consider that there are other factors contributing to malignant transformation of a craniopharyngioma.
Figure 6: Stereotactic radiotherapy dose planning (total dose 60 Gy in 30 fractions).
Human GH is a classical pituitary endocrine hormone essential for normal postnatal growth that has pleiotropic effects across multiple physiological systems, while its overproduction has been linked to cancer. The hormone is also expressed in extra-pituitary tissues and shows localized autocrine/paracrine effects at those sites [10]. Although the theoretical concern of increased tumor growth for patients taking GH has been addressed by numerous studies, a meta-analysis by Shen et al. included 4 studies and actually found that GH was associated with a decreased risk of craniopharyngioma recurrence in adults [11].
The present patient received GH replacement therapy to compensate for severe deficiency at 24 years after undergoing craniopharyngioma removal with no radiotherapy performed for the residual tumor, and gradual tumor growth was noted after GH administration. Although the precise cause of progression is unknown, GH might have been a factor in the present case of malignant transformation; thus, careful attention should be paid for rapid tumor growth in patients with a residual tumor who undergo GH replacement therapy. Once the tumor shows transformation to malignancy, it can continue to grow even if GH replacement therapy is stopped, with tumor mass removal necessary to confirm the histopathology for determining adjuvant therapy.
Conclusion
Continuous hormone exposure associated with GH replacement therapy may lead to malignant transformation of a craniopharyngioma. Close attention to follow-up imaging findings is necessary to detect change or rapid tumor growth following exposure to GH.
Conflicts of Interest
None.
Funding
None.
Abbreviations
WHO: World Health Organization
SRT: stereotactic radiotherapy
GH: growth hormone
Gd: gadolinium
Gy: gray
MRI: magnetic resonance imaging
CT: computed tomography
Article Info
Article Type
Case ReportPublication history
Received: Wed 26, Feb 2020Accepted: Sat 07, Mar 2020
Published: Fri 13, Mar 2020
Copyright
© 2023 Fumihiko Nishimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CRSS.2020.01.04
Author Info
Fumihiko Nishimura Hiroyuki Nakase Ichiro Nakagawa Kentaro Tamura Miho Kakutani Ryosuke Matsuda Shuichi Yamada Yasuhiro Takeshima Yasushi Motoyama Yoshiaki Takamura Young-Soo Park
Corresponding Author
Fumihiko NishimuraDepartment of Neurosurgery, Nara Medical University, Kashihara City, Nara, Japan
Figures & Tables
References
- Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F et al. (1998) The descriptive epidemiology of craniopharyngioma. J Neurosurg 89: 547-551. [Crossref]
- Mark RJ, Lutge WR, Shimizu KT, Tran LM, Selch MT et al. (1995) Craniopharyngioma: treatment in the CT and MR imaging era. Radiology 197: 195-198. [Crossref]
- Rodriguez FJ, Scheithauer BW, Tsunoda S, Kovacs K, Vidal S et al. (2007) The spectrum of malignancy in craniopharyngioma. Am J Surg Pathol 31: 1020-1028. [Crossref]
- Jeong TS, Yee GT, Kim NR (2017) Malignant transformation of craniopharyngioma without radiation therapy: case report and review of the literature. J Korean Neurosurg Soc 60: 108-113. [Crossref]
- Sofela AA, Hettige S, Curran O, Bassi S (2014) Malignant transformation in craniopharyngioma. Neurosurgry 75: 306-314. [Crossref]
- Akachi K, Takahashi H, Ishijima B, Nakamura Y, Oda M et al. (1987) Malignant changes in a craniopharyngioma. No Shinkei Geka 15: 843-848.
- Negoto T, Sakata K, Aoki T, Orito K, Nakashima S et al. (2015) Sequential pathological changes during malignant transformation of craniopharyngioma: a case report and review of the literature. Surg Neurol Int 6: 50. [Crossref]
- Chunhui L, Chuzhong L, Zhenye L, Yilin S, Yazhuo Z (2016) Malignant transformation of radiotherapy-naϊve craniopharyngioma. World Neurosurg 88: 690e1-690e5.
- Narla S, Govindraj J, Chandrasekar K, Sushama P (2017) Craniopharyngioma with malignant transformation: review of literature. Neurol India 65: 418-420.[Crossref]
- Lu M, Flanagan JU, Langley RJ, Hay MP, Perry JK (2019) Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther 4: 3. [Crossref]
- Shen L, Sun CM, Li XT, Liu CJ, Zhou YX (2015) Growth hormone therapy and risk of recurrence/progression in intracranial tumors: a meta-analysis. Neurol Sci 36: 1859-1867. [Crossref]
