Luteinizing Hormone and Ovarian Stimulation for In-Vitro Fertilization: Do Science and Business Always Agree?
A B S T R A C T
The current commentary paper follows the historical introduction of gonadotropins and gonadotropin releasing hormone (GnRH) analogues to the in-vitro fertilization (IVF) market. We maintain that business decisions significantly influenced research and development; however, pharma decisions did not always align with physiology and clinical interests. Specifically, the never-ending debate on the issue of luteinizing hormone (LH) supplementation during ovarian stimulation was repeatedly studied using population-based randomized controlled trials. However, LH activity supplementation is an endocrine issue and therefore, specific endocrine inclusion/exclusion criteria should be used when assessing the needs or not for LH in our “every-day” patients. We propose that the approach until now has defocused the research question and thus, also the debate and that there is a need to revisit physiology and clinical thinking if the LH supplementation issue is to be unravelled.
Keywords
IVF, gonadotropins, GnRH analogs, LH supplementation, endocrinology
Introduction
Fertility treatments witnessed a technological breakthrough in the last 2 decades of the 20th century, particularly the development of IVF. The parallel development of fertility medications allowed large-scale, global adoption of fertility technology. In this opinion paper we follow the major steps in the development of these medications. We propose that business considerations by the Pharma industry along the way did not always agree with clinical interests. In this light, we propose an alternative approach to LH supplementation during the ovarian stimulation question. Specifically, we maintain that population-based studies may be replaced with studies that consider specific endocrine events that may expose the individual patient to transient LH deficiency and the need for LH supplementation.
Human Menopausal Gonadotropin
Human menopausal gonadotropin (hMG) was introduced into clinical use by Bruno Lunenfeld in 1961 and quickly revolutionized fertility treatment [1]. The product, Pergonal®, contained follicle stimulating hormone (FSH) and LH activity in a 1:1 ratio. With the exponential growth of global IVF treatment cycles in the 1990's, and a limited high-quality menopausal urinary FSH supply, it became imperative to secure supply and quality by implementing recombinant DNA technology to produce recombinant human gonadotropins. The natural step forward would have been the production of “recombinant Pergonal”, i.e., a product containing recombinant human FSH and recombinant human LH, however, this did not materialize, and instead, the race to market resulted in the introduction of Follitropin alpha (Gonal-F®) by Serono and Follitropin beta (Puregon®) by Organon, both products containing recombinant human FSH, only. The two companies decided to invest their efforts and resources in producing only one gonadotropin molecule (FSH), leaving recombinant LH production “for the future”, and a natural question to ask, taking physiology into account, is why was LH left for the future? We suggest that the answer had to do with another Pharma industry decision, namely with the production of GnRH analogues.
GnRH Analogues
In 1977, the Nobel Prize in Physiology and Medicine was divided between Rosalyn Yalow ‘for the development of RIAs of peptide hormones’ and the other half jointly to Roger Guillemin and Andrew Victor Schally ‘for their discoveries concerning the peptide hormone production of the brain’. This discovery of GnRH led to a race to find potential analogues. The natural step forward would have been the production of a GnRH antagonist for the prevention of a premature LH surge and early luteinization during ovarian stimulation, however, again, this did not materialize. It turned out that it was easier and quicker to produce GnRH agonist preparations for clinical use, and thus, GnRH agonist (GnRHa) products were registered for pituitary down-regulation in IVF, whereas the development of GnRH antagonists was “left for the future”. Indeed, the from a patient perspective inconvenient “long GnRHa down-regulation” protocol quickly became the gold standard protocol in IVF, since it eliminated cycle cancellations caused by spontaneous ovulation, leading to significantly improved success rates and facilitating the scheduling of the treatment. This occurred in spite of the fact that the long GnRHa down-regulation protocol was cumbersome and deviated significantly from physiology; however, this was as “good as it got” and we did not have any other options at the time.
Which Gonadotropin Formulation to Use in the Long GnRHa Down-Regulation Protocol?
With the long GnRHa down-regulation protocol well established globally, the need for LH activity supplementation during ovarian stimulation was explored and questioned. Urinary FSH preparations were introduced and reported to be equal, or even better, than the old HMG products [2-5]. These and similar publications added scientific justification to the decision of the Pharma industry to introduce recombinant FSH products only, claiming that LH was not needed in the context of GnRHa down-regulation and ovarian stimulation, although physiology tells us that LH and FSH work in synergy, playing complementary roles during follicle development and ovulation.
GnRH Antagonists
GnRH antagonists were introduced to the market in early 2000, and after a decade, the GnRH antagonist protocol almost totally replaced the long GnRHa down-regulation protocol. It was convenient for Pharma and clinicians alike to transfer the knowledge and experience from the long GnRHa down-regulation protocol to the GnRH antagonist protocol and continue the use of recombinant FSH preparations for ovarian stimulation. However, the endocrinology of the GnRH antagonist protocol is totally different from that of the GnRHa long down-regulation protocol and the natural cycle [6].
Thus, according to the two cell- two gonadotropin concept theca cell-derived, LH-dependent, aromatizable androgens (mainly androstenedione) are converted into E2 by FSH-induced granulosa cell aromatase activity. The extent of aromatase activity is limited by the amount of precursor available, which in turn depends on LH levels. During the natural cycle, LH levels are high, ranging between 6-8 IU/l during the follicular phase, which allows for a sufficient supply of androgens, for a continuous rise in E2 levels, determined by the growing number of granulosa cells of the dominant follicle, and a parallel increase in aromatase activity [7].
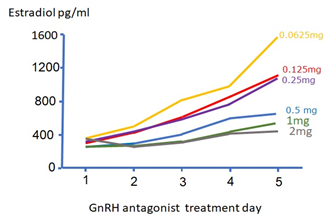
When comparing the GnRH antagonist protocol to the long GnRHa protocol, we suggest that a sharp drop in LH – as seen during GnRH antagonist treatment - will cause a sudden decrease in androgen precursor availability, resulting in an insufficient E2 production by the growing follicles, manifested in a drop or plateauing in circulating E2 levels. Moreover, as androgens play a pivotal role in folliculogenesis in terms of primary growth via synergy with insulin growth factor (IGF) 1, and FSH receptor induction on granulosa cells, stagnation of follicular growth is seen in sub-sets of patients who benefit from of LH supplementation during GnRH antagonist co-treatment [8]. Thus, E2-plateauing and follicular stagnation are typical signs of an iatrogenic – or acquired LH deficiency (Figure 1) [9]. We suggest that, at least in part, the reason for “hypo-response to controlled ovarian stimulation” and “initial slow response or stagnation in follicle growth during ovarian stimulation with FSH monotherapy" is caused by an iatrogenic, GnRH antagonist-mediated, LH deficiency [10].
Figure 1: Modified from the Ganirelix dose-finding study. After 5 days of recombinant FSH ovarian stimulation, most patients reached an E2 level of about 400 pg/ml. In contrast, after Ganirelix dosing, patients who received the highest dosing (1 mg and 2 mg) have E2 stagnation during the rest of stimulation period.
In comparison, during the long GnRHa down-regulation protocol, LH levels are significantly lower as compared to during GnRH antagonist co-treatment, and with minimal fluctuations over time, allowing follicles to adjust to the down-regulated LH levels [11]. In the majority of these patients, unless LH is completely eliminated, a steady rise in E2 levels and an increase in follicular growth during stimulation is observed depending on the amount of exogenous FSH supplied to the system. Theoretically, the actual LH level plays a minor role in the majority of patients since only part of follicular LH receptors need to be activated to obtain maximal steroidogenic response and growth of the follicle [12].
However, from a physiological point of view, it seems plausible that it takes some time for the aromatase system to adjust to a given low circulating endogenous LH level. Hence, the conclusion made from GnRHa down-regulation: "...there is little underlying physiological support for the addition of LH in stimulation protocols", contrasted by others who suggested that 12-14 % of GnRHa down-regulated patients benefitted from LH supplementation [11, 13].
In the GnRH antagonist-based cycle, following a mild natural decrease in LH levels during the first 5 days of stimulation, a sudden GnRH antagonist-mediated LH drop leads to depleted E2 biosynthesis and follicular growth stagnation. We, therefore, suggest that the sharp drop in LH level is clinically significant, rather than the absolute level itself, based on the lessons learned from the Ganirelix dose-finding study [14]. Of note, the Ganirelix dose-finding study, as usual, recruited optimal “model” patients in terms of age, ovulatory pattern, body mass index (BMI), and ovarian morphology. However, "model" patients comprise no more than one-third of “real life” patients [15].
Even in the “model” patient population, the response of the pituitary to the chosen GnRH antagonist dose (0.25 mg) will obey a “bell-shape” response curve. Some patients may “hypo-respond” to the 0.25 mg dose and will be at risk of a premature LH rise, while other patients may “hyper-respond” to the 0.25 mg dose, being at risk of LH over- suppression. In reality, a GnRH antagonist dose-response scatter of the 0.25 mg gold standard dose was never published. Instead, it may have been convenient to the Pharma industry to once again come up with a “one dose fits all” model, ignoring potential individual physiological abnormal responses, and ignoring the majority of patients who were excluded from the dose-finding study as they were not “model” patients. While the issue of individual gonadotropin dosing for follicular stimulation has been intensively studied, the question of an individual GnRH antagonist dosing regimen has been conveniently put aside or ignored.
Recombinant LH
Recombinant human LH (rLH) was initially introduced to the market in the 2000's, and the idea was to use rLH for ovulation triggering; however, the project was halted due to the large dose of rLH needed to induce optimal follicular maturation, which introduced financial as well as clinical considerations [16]. Instead, going back to the old Pergonal® days, ignoring the twists and turns summarized above, but remembering physiology, a straightforward implementation of a “recombinant Pergonal” preparation principle was introduced alongside the nowadays gold standard GnRH antagonist protocol.
Population-Based Randomized Controlled Trial (RCT)'s and the Supplemented LH Question
So far, the question of adding LH in ovarian stimulation before IVF has been approached by numerous RCT's. The inclusion and exclusion criteria in these RCT's were population-based, in line with previous large RCT's used to compare different gonadotropin products, as required by regulatory agencies for product registration and marketing.
Using this approach to study an endocrine question, like adding LH in ovarian stimulation for IVF, cannot be justified. A population-based RCT is likely to miss patients who might benefit from LH supplementation. We argue that when assessing an endocrine question, we need to base RCT's on specific endocrine inclusion/exclusion criteria. Based on the Ganirelix dose-finding study, the following two endocrine events could identify patients who might benefit from LH supplementation:
i. The endogenous LH level just before GnRH antagonist dosing
ii. The recovery of endogenous LH 24 hours post-dosing
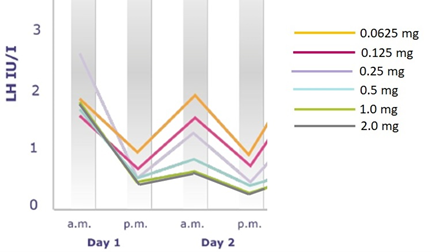
Figure 2: Modified from the Ganirelix dose-finding study. All patients were stimulated with recombinant FSH. Day 1 is the first day of antagonist dose given at 8 am, followed by a repeated LH blood testing 8 hours later. The magnitude of the LH drop 8 hours after Ganirelix dosing is largest when pre-dosing serum LH is highest. Eight hours post Ganirelix dosing LH is <1.0 IU/I with all doses used, however, LH recovery 24 hours post dosing is different between the doses used. Of note, patients receiving the highest Ganirelix doses (1 mg and 2 mg) had very low LH levels on the day of trigger and also poor reproductive outcomes.
Figure 2 shows that the LH drop was highest when LH was highest before dosing, even if the system was exposed to the moderate 0.25 mg dose. Also, it is clear that the difference between the higher doses (1 and 2 mg, leading to poor reproductive outcomes) and the 0.25 mg dose was not apparent 8 hours after dosing, but 24 hours after dosing (LH recovery).
These above mentioned endocrine events were previously explored in 50 GnRH antagonist co-treated patients. Thus, in the morning of the first GnRH antagonist bolus, a blood sample was performed for LH, E2 and progesterone prior to GnRH antagonist administration. Twenty-four hours after the first GnRH antagonist injection, another blood sample was performed. If the LH level was less than 50% of the level measured 24 hours earlier, the subject was defined as “over-suppressed” to the 0.25 mg dose, and a daily dose of 150 units of recombinant LH (lutropin alpha; Luveris®, Merck Serono, Herzliya, Israel) was added from that day onwards (in parallel to follitropin alfa) until ovulation trigger. Importantly, a total of 26% of patients were “over-suppressed”, and these patients also demonstrated a significant decrease in estradiol levels during the first 24 hours after the initial GnRH antagonist administration; however, LH “rescue” resulted in not only a non-significant difference in estradiol levels on the day of ovulation trigger but also a non-significant difference in FSH consumption when compared to normal responders to the GnRH antagonist. Moreover, no difference was seen in oocyte and embryo number or reproductive outcome [17]. To corroborate the findings of this pilot study clearly, a large-scale RCT is warranted in the “over-suppressed” patient category, however, this would be the optimal way to explore the “LH-question” in an endocrine and objective way.
Outlook
Pharma industries and business decisions significantly influence the way we practice ovarian stimulation in IVF. Product development often results in financial and practical considerations, deviating from physiology. In IVF, when considering ovarian stimulation, LH supplementation, and use of the GnRH antagonist protocol, science and business do definitely not agree. We suggest that we revisit physiology and clinical thinking and individualize our treatment strategy according to the needs of our "every-day" patients.
Funding
None.
Author Contributions
Conceptualization, writing by both authors. Both authors have read and agreed to the published version of the manuscript.
Ethical Approval
None.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
None.
Abbreviations
LH: Luteinizing Hormone
IVF: In Vitro Fertilization
GnRH: Gonadotropin Releasing Hormone
hMG: Human Menopausal Gonadotropin
FSH: Follicle Stimulating Hormone
E2: Estradiol
BMI: Body Mass Index
IGF: Insulin Growth Factor
rLH: Recombinant Human LH
RCT: Randomized Controlled Trial
Article Info
Article Type
CommentaryPublication history
Received: Sat 09, Jul 2022Accepted: Mon 25, Jul 2022
Published: Mon 22, Aug 2022
Copyright
© 2023 Shahar Kol. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CEI.2022.01.01
Author Info
Corresponding Author
Shahar KolIVF Unit, Elisha Hospital, Haifa, Israel
Figures & Tables
References
1. Lunenfeld B, Rabau E, Rumney G,
Winkelsberg G (1961) The responsiveness of the human ovary to gonadotrophin
(Hypophsis III). Proc Third World Congress Gynecology and Obstetrics, Vienna 1:
220.
2. Bagratee JS, Lockwood G, Bernal AL,
Barlow DH, Ledger WL (1998) Comparison of highly purified FSH (metrodin-high
purity) with pergonal for IVF superovulation. J Assist Reprod Genet
15: 65-69. [Crossref]
3. Westergaard LG, Erb K, Laursen S,
Rasmussen PE, Rex S (1996) The effect of human menopausal gonadotrophin and
highly purified, urine-derived follicle stimulating hormone on the outcome of
in-vitro fertilization in down-regulated normogonadotrophic women. Hum
Reprod 11: 1209-1213. [Crossref]
4. Balasch J, Fábregues F, Creus M,
Moreno V, Puerto B et al. (1996) Pure and highly purified follicle-stimulating
hormone alone or in combination with human menopausal gonadotrophin for ovarian
stimulation after pituitary suppression in in-vitro fertilization. Hum
Reprod 11: 2400-2404. [Crossref]
5. Mercan R, Mayer JF, Walker D, Jone
S, Oehninger S et al. (1997) Improved oocyte quality is obtained with follicle
stimulating hormone alone than with follicle stimulating hormone/human
menopausal gonadotrophin combination. Hum Reprod 12: 1886-1889. [Crossref]
6. Knobil E (1974) On the control of
gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res 30:
1-46. [Crossref]
7. Abraham GE, Odell WD, Swerdloff RS,
Hopper K (1972) Simultaneous radioimmunoassay of plasma FSH, LH, progesterone,
17-hydroxyprogesterone, and estradiol-17 beta during the menstrual cycle. J
Clin Endocrinol Metab 34: 312-318. [Crossref]
8. Weil S, Vendola K, Zhou J, Bondy CA
(1999) Androgen and follicle-stimulating hormone interactions in primate
ovarian follicle development. J Clin Endocrinol Metab 84: 2951-2956. [Crossref]
9. Bosch E, Alviggi C, Lispi, M,
Conforti A, Hanyaloglu AC et al. (2021) Reduced FSH and LH action: implications
for medically assisted reproduction. Hum Reprod 36: 1469-1480. [Crossref]
10. Conforti A, Esteves SC, Di Rella F,
Strina I, De Rosa P et al. (2019) The role of recombinant LH in women with
hypo-response to controlled ovarian stimulation: a systematic review and
meta-analysis. Reprod Biol Endocrinol 17: 18. [Crossref]
11. Peñarrubia J, Fábregues F, Creus M,
Manau D, Casamitjana R et al. (2003) LH serum levels during ovarian stimulation
as predictors of ovarian response and assisted reproduction outcome in
down-regulated women stimulated with recombinant FSH. Hum Reprod 18:
2689-2697. [Crossref]
12. Chappel SC, Howles C (1991)
Reevaluation of the roles of luteinizing hormone and follicle-stimulating
hormone in the ovulatory process. Hum Reprod 6: 1206-1212. [Crossref]
13. De Placido G, Alviggi C, Perino A,
Strina I, Lisi F et al. (2005) Recombinant human LH supplementation versus
recombinant human FSH (rFSH) step-up protocol during controlled ovarian
stimulation in normogonadotrophic women with initial inadequate ovarian response
to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod
20: 390-396. [Crossref]
14. The Ganirelix Dose-Finding Study
Group (1998) A double-blind, randomized, dose finding study to assess the
efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org
37462) to prevent premature luteinizing hormone surges in women undergoing
ovarian stimulation with recombinant follicle stimulating hormone (Puregon).
The ganirelix dose-finding study group. Hum Reprod. 13: 3023-3031. [Crossref]
15. Hershkop E, Segal L, Fainaru O, Kol
S (2017) Model' versus 'everyday' patients: can randomized controlled trial
data really be applied to the clinic? Reprod Biomed Online 34: 274-279.
[Crossref]
16. European Recombinant LH Study Group (2001) Human recombinant luteinizing hormone is as effective as, but safer than, urinary human chorionic gonadotropin in inducing final follicular maturation and ovulation in in vitro fertilization procedures: results of a multicenter double-blind study. J Clin Endocrinol Metab 86: 2607-2618. [Crossref]
17. Kol S (2014) Individualized Treatment from Theory to Practice: The Private Case of Adding LH during GnRH Antagonist-based Stimulation Protocol. Clin Med Insights Reprod Health 8: 59-64. [Crossref]
