Long-Term Remissions in Patients with Peripheral T-Cell Lymphoma Following High-Dose Chemotherapy, Autologous Stem Cell Transplantation (ASCT) and Subsequent Radiotherapy
A B S T R A C T
Purpose: The prognosis of patients with peripheral T-cell lymphoma and missing complete remission or relapsing before ASCT is assessed to be unfavourable. The aim of this study is to contribute data to the open question, whether additional radiotherapy improves the outcome.
Patients and Methods: Forty-eight patients with peripheral T-cell-lymphoma were treated in our institution with high-dose therapy (usually BEAM protocol) and ASCT (age median 54 years, range 19-77). Twenty-five patients received ASCT in first treatment line, 13 in second line, 10 in third line (all refractory to 2nd-line salvage therapy). Seven of these 48 patients received radiotherapy (36-40 Gy, median 36) after ASCT.
Results: Five-year overall survival (OS) and progression-free survival (PFS) after 1st-line ASCT and 2nd-line ASCT were 59% and 50%, respectively, and after 3rd-line ASCT significantly lower with 30% and 30%, respectively. Twenty-one patients achieved a sustained complete remission of 2.7 - 22.7 years (median 5.8). No patient relapsed after radiotherapy.
Conclusion: Sustained long-term remissions can be achieved in patients with peripheral T-cell lymphoma following ASCT in first, second or third treatment line. Early irradiation after ASCT can possibly consolidate remission in localized residual or refractory disease.
Keywords
Autologous stem-cell transplantation, radiotherapy, peripheral T-cell lymphoma, long-term remission
Highlights
As PTCL are assessed as lymphomas with poor prognosis, we have analyzed the results of our 48 patients treated with autologous stem cell transplantation (ASCT). We observed 21 long-term remissions (up to 22 years) and especially no relapse in 7 patients treated with radiotherapy of resistant or remaining active lymphoma after ASCT. Although the group of irradiated patients is rather small, this finding is an interesting supplement to our findings in early relapsed aggressive B-cell lymphomas treated with a very similar approach, showing that radiotherapy after ASCT can consolidate remissions. This observation is of special interest as up to now only a few data were published in this field.
Introduction
High-dose therapy (HDT) with autologous stem cell transplantation (ASCT) is known as an efficacious treatment which can achieve long-term remissions in patients with peripheral T-cell lymphoma [1-2]. But the prognosis of patients with peripheral T-cell lymphoma without reaching a complete remission appears to be unfavourable following ASCT [3-4]. So the impact of ASCT in the management of patients with peripheral T-cell lymphomas remains unclear [5-29]. The analysis of the relapse sites after ASCT in B-cell lymphomas concluded that local recurrences are a significant problem and support the incorporation of consolidative radiotherapy (RT) as a part of salvage treatment [30-31]. Several studies have been published on the combination of chemotherapy and radiotherapy also in special subgroups of peripheral T-cell, but rarely in combination with ASCT [32-41]. So far it is an open question whether additional RT is able to improve the outcome. An exception is extranodal nasal-type natural killer/T Cell lymphoma, for which radiotherapy is a part of the standard therapy. Therefore, we analyzed our follow-up data of patients with a peripheral T-cell lymphoma, mainly after high-dose chemotherapy with the BEAM protocol and ASCT, combined with subsequent RT of refractory or residual disease, if feasible.
Design and Methods
Fifty-nine patients with the diagnosis of a peripheral T-cell lymphoma were referred to our institution and treated with chemotherapy. Because of early lymphoma progression or very poor performance status only 48 of these patients received subsequently high-dose therapy and ASCT between September 1996 and April 2020. All these patients were identified by the electronic hospital information system and the documentation system of our ASCT patients. The data were analyzed retrospectively. The diagnoses were confirmed by experienced reference hematopathologists only. All patients were treated under the responsibility of the authors. Several patients participated in prospective trials: six patients within the mega-CHOEP trial, three patients within a special T-cell lymphoma trial [9, 10, 17-19]. Some patients were treated with ASCT analog to the third trial for the first-line treatment: the AATT trial, following the termination of the patient recruitment of this study [8]. A relapse was confirmed histologically if feasible. Patients with ALK positive anaplastic large cell lymphoma (n=5) were treated with ASCT if they had additional unfavourable prognostic factors: three patients with 1st-line ASCT and a chemotherapy pretreatment of a hodgkin´s lymphoma, with only partial remission before ASCT or with an IPI with high-intermediate risk, respectively.
The other two patients received a 2nd-line ASCT. The patient characteristics of 48 patients treated with ASCT are shown in (Table 1). For the salvage protocol before ASCT, most of the patients received two to three courses of the DHAP protocol (with dexamethasone, high-dose cytarabine and cisplatin) or a similar protocol like ESHAP (with etoposide, high-dose prednisolone, high-dose cytarabine and cisplatin). In the case of missing response after two courses of these regimens, patients were assessed as refractory, as described in the SCHOLAR-1 study for diffuse large B-cell lymphomas, and the therapy was changed to the ICE (one to two courses with ifosfamide, carboplatin and etoposide) or a similar protocol [6]. Autologous stem cells were collected by apheresis in the blood donation service following these protocols. In the case of refractory disease, a donor search for potential allogeneic transplantation after ASCT was started. In compliance with the institutional guidelines for autologous SCT eligibility, all patients had to demonstrate appropriate cardiac function with an ejection fraction by echocardiogram of > 50% and no symptoms of congestive heart failure. Pulmonary function was demonstrated by a forced expired volume in the first second of > 50% and diffusion capacity of carbon monoxide of > 50% of predicted value. Patients were also required to have creatinine of < 2.5 x upper normal limit, bilirubin < 1.5 x upper normal limit and eastern cooperative oncology group (ECOG) performance status < 3 prior to transplant.
Table 1: Characteristics
of 48 patients with peripheral T-cell lymphoma after ASCT.
|
Patients,
n |
48 |
|
Age
(years, median/range) |
54
(19-77) |
|
Sex
(female/male), n (%) |
16
(33) / 32 (67) |
|
Histology: Anaplastic Large-Cell Lymphoma, 13 of whom ALK-, 6
ALK+, 2 ALK not tested Peripheral T-Cell Lymphoma
(NOS and similar) Angioimmunoblastic T-Cell
Lymphoma Enteropathy-Associated
T-Cell Lymphoma Other T-cell lymphomas, n
(%) Stage
(Ann Arbor) 1st-line ASCT: I (enteropathy-associated TL)
II
III IV IPI
at 1st-line chemotherapy: low risk
low-intermediate risk
high-intermediate risk high risk |
21
(44)
12
(25) 11
(23) 2 (4) 2 (4) 1
(4) 2 (8) 11
(44) 11
(44) 7 (28) 11
(44) 4 (16) 1 (4) |
|
Prior
treatment with anthracyclines (CHOP or CHOEP), n (%) |
48
(100) |
|
High-dose
therapy: BEAM Mega-CHOEP
TBI/Cyclophosphamide Treatment
line: 1st-line ASCT, n (%) 2nd-line
ASCT, n (%) 3rd-line
ASCT, n (%) Time
from initial diagnosis to 1st relapse in 2nd-line ASCT,
median/range (mo) |
40
(83) 6 (13) 2 (4) 25
(52) 13
(27) 10
(21) 16
(2-123) |
|
Time
from initial diagnosis to 1st relapse in 3rd-line ASCT,
median/range (mo) Refractory
to 2nd-line chemotherapy, n (%) Second
relapse, n (%) LDH:
elevated at 1st-line ASCT, n (%),
elevated at 2nd-line
ASCT, n (%), elevated at 3rd-line
ASCT, n (%), no information at 3rd-line
ASCT |
7
(1-44) 10
(21) 0 1
(4) 1
(8) 4
(40) 1
(10) |
|
Remission
status: CR/PR/SD/PD at 1st-line ASCT, n
CR/PR/SD/PD/ED/NI after 1st-line ASCT, n CR/PR/SD/PD at
2nd-line ASCT, n CR/PR/SD/PD/ED
after 2nd-line ASCT, n CR/PR/SD/PD at
3rd-line ASCT, n CR/PR/SD/PD/ED
after 3rd-line ASCT, n |
4/21/0/0 14/8/0/1/1/1 8/5/0/0 10/1/0/1/1 0/1/5/4 3/1/1/3/2 |
|
Radiotherapy
after 1st-line ASCT, n (%) Radiotherapy
after 2nd-line ASCT, n (%) Radiotherapy
after 3rd-line ASCT, n (%) Allogeneic
stem cell transplantation after 1st-line ASCT, n (%) |
3
(12) 2
(15) 2
(20) 4
(16) |
ALK:
Anaplastic Lymphoma Kinase; TL: T-cell Lymphoma; NOS: Not Otherwise Specified;
IPI: International Prognostic Index; BEAM: High-dose BCNU, Etoposide,
Cytarabine, Melphalan; Mega-CHOEP: High-dose Cyclophosphamide, Doxorubicin,
Vincristine, Etoposide, Prednisone; TBI: Total Body Irradiation; mo: Months;
LDH: Lactic Dehydrogenase; CR: Complete Remission; PR: Partial Remission; SD:
Stable Disease; PD: Progressive Disease; ED: Early Death; NI: No Information.
All patients provided informed consent to undergo autologous SCT and allowed their clinical records to be reviewed for research purposes. The above-mentioned BEAM protocol consisted of carmustine (300 mg/m2, in patients with an age over 65 years 200 mg/m2) on day -6, etoposide (200 mg/m2) on days -5 to -2, cytarabine (200 mg/m2 twice a day) on days -5 to -2, and melphalan (140 mg/m m2) on day -1. Peripheral-blood stem cells were reinfused on day 0, at least 24 hours after completion of BEAM. Altogether, 25 patients received ASCT in 1st treatment line, 13 in 2nd line and 10 in 3rd line (all 10 refractory to 2nd-line salvage therapy). PET-CT examinations were carried out in only 8 patients after ASCT due to problems with reimbursement. The remaining patients received a computer tomography, 13 of whom in complete remission. Patients with refractory disease before ASCT or residual masses after ASCT, received radiotherapy with 36-40 Gy (median 36 Gy) with a single dose of 1.8 to 2 Gy five times per week, within the field of persisting lymphoma, if feasible. Patients with disseminated organ involvement could not be irradiated in these localisations. Response assessment was performed by careful clinical examination combined with abdomen ultrasound and chest x-ray (and partly CT) at regular three-monthly intervals in the first two years after ASCT, every six months in the following three years and every 12 months thereafter. Relapses after ASCT were diagnosed by medical imaging (with increasing lymphoma masses, assessed by CT), partly by histology. The protocol was approved by the medical ethics committee of the university Oldenburg and carried out in accordance with the Declaration of Helsinki.
Statistical Analysis
Overall survival (OS) was defined as time from ASCT to death from any cause. Progression-free survival (PFS) was defined as time from ASCT to relapse of T-cell lymphoma, progression or death from any cause. Relapse/progression was defined as appearance of new lesions and/or an increase of known lesions of >25%. Definition of refractory disease for this evaluation: the disease was called refractory, if no remission after two courses of salvage therapy (like R-DHAP) could be reached before ASCT. Second-line ASCT was defined as ASCT after first T-cell lymphoma relapse or refractory disease after induction therapy (first therapy regimen). Third-line ASCT was defined as ASCT after refractory disease after salvage therapy following first relapse. “Remaining masses” after ASCT were defined as lymphoma residuals after ASCT described by CT scan.
Survival times were estimated using the kaplan-meier method. Survival curves for different strata were compared by the log-rank test. Uni- and multivariate cox proportional hazards regression was performed with OS and PFS, respectively, as the dependent variable and the explanatory variables listed in (Table S2). Significance levels were set at 0.05. Statistical parameters were calculated in R (version 4.2.2). Data were analysed as of 27th December 2022.
Results
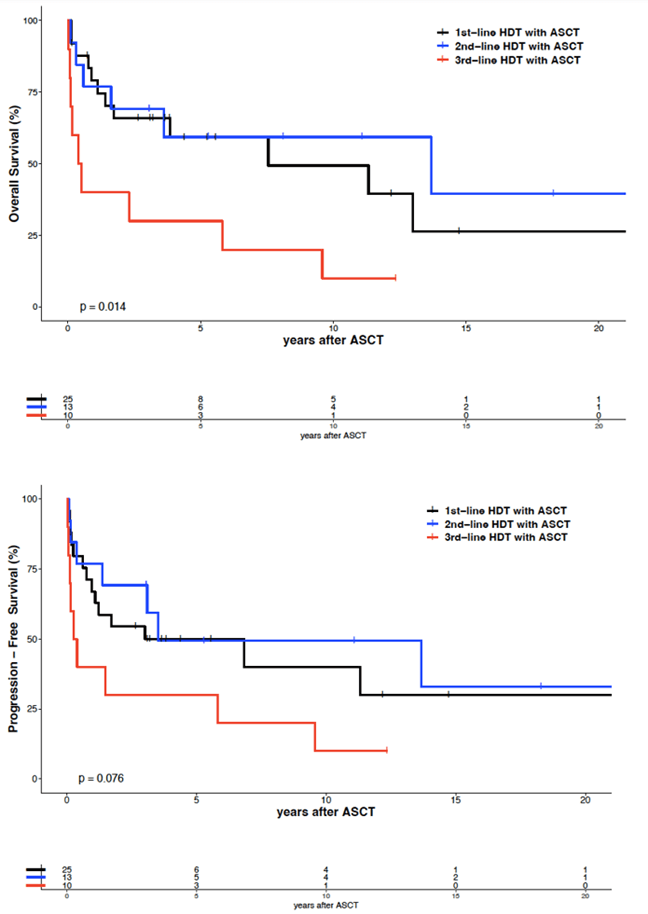
The median follow-up was 5.3 years for the 21 surviving patients (range 0.2 - 22.8 years). Twenty-one of 48 patients relapsed 1-82 (median 7) months after ASCT. No relapses occurred after 37 months following ASCT in 2nd or 3rd remission, except for one patient 42 months after 2nd-line ASCT (Figure 2). Twenty-one patients achieved a sustained complete remission of 2.7 - 22.7 years (median 5.8). Their characteristics are shown in (Table 2). Five-year and ten-year overall survival (OS) after 1st-line ASCT were 59% and 49%, respectively. Five-year and ten-year progression-free survival (PFS) after 1st-line ASCT were 50% and 40%, respectively. Five-year and ten-year OS after 2nd-line ASCT were 59% and 59%, respectively. Five-year and ten-year PFS after 2nd-line ASCT were 50% and 50%, respectively, without any significant difference between 1st-line and 2nd-line ASCT. However, 5-year and 10-year OS after 3rd-line ASCT were significantly lower with 30% and 30%, respectively (p=0.009). Five-year and ten-year PFS after 3rd-line ASCT were 30% and 10%, respectively, but without statistically significant difference compared to 1st-line ASCT. The median OS and PFS after 1st-line ASCT were 7.6 and 3.0 years, respectively, after 2nd-line ASCT 13.7 and 3.5 years and after 3rd-line ASCT 0.5 and 0.3 years, respectively.
Table 2: Characteristics
of 21 patients with peripheral T-cell lymphoma and long-term remission after ASCT.
|
Patients,
n |
21 |
|
Age
(years, median/range) |
54
(19-73) |
|
Sex
(female/male), n (%) |
8
(38) / 13 (62) |
|
Histology: Anaplastic Large-Cell Lymphoma, 5 of whom ALK-, 5
ALK+, 2 ALK not tested, n (%) Peripheral T-Cell Lymphoma
(NOS and similar), n (%) Angioimmunoblastic T-Cell
Lymphoma, n (%) Enteropathy-Associated
T-Cell Lymphoma, n (%) Stage
(Ann Arbor) 1st-line ASCT: I (enteropathy-associated TL), n (%)
II, n (%) III, n (%)
IV, n (%) IPI
at 1st-line chemotherapy: low risk, n (%)
low-intermediate risk, n (%) high-intermediate risk,
n (%)
high risk, n (%) |
12
(57) 4(17) 4
(17) 1
(5) 1
(5) 3
(14) 5
(24) 2
(10) 7
(33) 2(10) 1 (5) 1 (5) |
|
Prior
treatment with anthracyclines (CHOP or CHOEP), n (%) |
21
(100) |
|
High-dose
therapy: BEAM Mega-CHOEP
TBI/Cyclophosphamide Treatment
line: 1st-line ASCT, n (%) 2nd-line
ASCT, n (%) 3rd-line
ASCT, n (%) Time
from initial diagnosis to 1st relapse in 2nd-line ASCT,
median/range (mo) |
20
(95) 0 1 (5) 11
(52) 7
(33) 3
(14) 25
(4-123) |
|
Time
from initial diagnosis to 1st relapse in 3rd-line ASCT,
median/range (mo) Refractory
to 2nd-line chemotherapy, n (%) Second
relapse, n (%) LDH:
elevated at 1st-line ASCT, n (%),
elevated at 2nd-line
ASCT, n (%), elevated at 3rd-line
ASCT, n (%), |
5
(1-9) 3
(14) 0 1
(9) 1
(14) 0 |
|
Remission
status: CR/PR/SD/PD at 1st-line ASCT, n CR/PR/SD/PD
after 1st-line ASCT, n CR/PR/SD/PD at
2nd-line ASCT, n CR/PR/SD/PD/ED
after 2nd-line ASCT, n CR/PR/SD/PD at
3rd-line ASCT, n
CR/PR/SD/PD/ED/NI after 3rd-line ASCT, n |
2/9/0/0 3/8/0/0 5/2/0/0 7/0/0/0 0/0/1/2 2/1/0/0 |
|
Radiotherapy
after 1st-line ASCT, n (%) Radiotherapy
after 2nd-line ASCT, n (%) Radiotherapy
after 3rd-line ASCT, n (%) Allogeneic
stem cell transplantation |
3
(27) 2
(29) 2
(67) 0 |
ALK:
Anaplastic Lymphoma Kinase; TL: T-cell Lymphoma; NOS: Not Otherwise Specified;
IPI: International Prognostic Index; BEAM: High-dose BCNU, Etoposide,
Cytarabine, Melphalan; Mega-CHOEP: High-dose Cyclophosphamide, Doxorubicin,
Vincristine, Etoposide, Prednisone; TBI: Total Body Irradiation; mo: Months;
LDH: Lactic Dehydrogenase; CR: Complete Remission; PR: Partial Remission; SD:
Stable Disease; PD: Progressive Disease; ED: Early Death; NI: No Information.
I Prognostic Factors
Multivariate analysis considering treatment line of ASCT showed a significant difference of OS between 1st-line and 3rd-line ASCT, but not between 1st-line and 2nd-line ASCT (Figure 1 & Table S2). An unfavourable prognostic factor for OS and PFS appears to be histology of angioimmunoblastic T-cell lymphoma, although the number of patients with this histologic subgroup was rather small (n=11). So, the impact of this observation should not be overestimated. This applies also to the result of the multivariate analysis that patients with an age of 60 years and above (n=15) have a better OS and PFS (p=0.014). Obviously, we have no data that could justify to withheld this kind of treatment from patients with a biological age up to 70 years. A further unfavourable prognostic factor was the kind of high-dose therapy regimen. The effectiveness of the sequential high-dose therapy with the mega-CHOEP regimen with a 5-year and 10-year OS of 33% and 17%, respectively, and a 5-year and 10-year PFS of 17% and 0%, respectively, in our cohort was low (Figure 3). Therefore, we did not use this regimen after the analysis of the trial [19]. The results of the mostly used BEAM regimen were significantly better with a 5-year and 10-year OS of 58% and 47%, respectively, and a 5-year and 10-year PFS of 50% and 41%, respectively. Two patients received TBI and cyclophosphamide within a trial, a regimen without any clear benefit for this lymphoma subgroup. Other variables (sex, LDH at ASCT, remission status at ASCT) showed no significant differences. So, patients reaching only a partial remission at ASCT appear to be qualified for this kind of treatment as well.
Figure 1: Overall survival and progression-free survival of 48 patients with peripheral T-cell lymphoma, by treatment line.
Figure 2: Overall survival and progression-free survival of 48 patients with peripheral T-cell lymphoma, by treatment with or without irradiation after ASCT.
Figure 3: Overall survival and progression-free survival of 48 patients with peripheral T-cell lymphoma, by HDT-regimen.
II Radiotherapy Following ASCT
Seven of 48 patients received radiotherapy of residual disease following ASCT (36 - 40 Gy, median 36 Gy), starting 5 -10 weeks (median 8 weeks) after ASCT: Three patients were irradiated with 36 Gy in the involved field after reaching a complete remission after ASCT, analog to earlier treatment protocols. Two patients received radiotherapy because of a positive PET-CT after ASCT. Two patients were irradiated because of lymphoma progression directly before ASCT in the progressive lymphoma area. No one of these seven patients suffered from a lymphoma relapse. The median remission duration amounts to 12.3 years (range 3.2 - 22.8). The 5-year and 10-year OS were 100% and 83%, respectively. The 5-year and 10-year PFS were identical with 100% and 83%, respectively (Figure 2). Two of these seven patients died with secondary invasive malignancies outside of the irradiation area: one woman with brain metastases of a small cell lung cancer 13.7 years after ASCT, one male patient with an esophageal cancer 5.8 years after ASCT. There was no evidence of grade 3 or 4 toxicities following radiotherapy. The characteristics of these seven patients are described in (Table S3 Supplement). They show no noteworthy accumulation of favourable prognostic factors. The multivariate analysis (Table S2 Supplement) revealed statistical difference between irradiated and not irradiated patients for PFS (p=0.024), not clearly for OS (p=0.08).
Why were only seven patients irradiated? Our earlier strategy to irradiate T-cell lymphoma patients with involved-field radiotherapy was changed shortly after 2001, as state of the art treatment protocols did not include radiotherapy after ASCT. After 2001, patients with complete remission at ASCT were no longer irradiated in our study. Due to reimbursement problems for PET-CT at that time, most patients with an unclear CT result with minimal questionable lymphoma remainings were controlled subsequently without further treatment. Patients not reaching a clear complete remission after ASCT usually relapsed and developed a poor performance status within short time, before radiotherapy could be organized.
Analysing the lymphoma relapse areas after ASCT could help to assess the impact of radiotherapy after ASCT. Given the details in (Table S1 Supplement) with many disseminated relapse areas, it can be suspected that only one patient would have benefitted from an involved-field irradiation. Together with three patients who had been irradiated within an involved field without a consecutive relapse, this means a benefit of probably four of 48 patients from an involved-field irradiation after ASCT, a small benefit that should not be ignored.
III Treatment of Relapse after ASCT
Five of 21 relapsed patients received an allogeneic stem cell transplantation. All five have died following this treatment due to transplant-related problems, two of them after a further lymphoma progression. Seven patients were treated with palliative chemotherapy, partly trying to reach a remission to carry out an allogeneic transplantation subsequently (without success). One patient received brentuximab and subsequent radiotherapy and remains in continuous remission (over 5 years). Six patients developed a very poor performance status and could not get any further lymphoma therapy. For two patients we did not get further information.
IV Non-Relapse Mortality and Second Invasive Malignancies
Two patients with an age of 50 and 58 years, respectively, died shortly (0.5 and 1.5 months, respectively) after ASCT in 3rd treatment line due to severe infection. Four patients deceased after the development of secondary invasive malignancies: non-small cell lung cancer (developed 4.0 years after 1st-line ASCT), small cell lung cancer (13.5 years after 2nd-line ASCT), acute myelogenous leukemia (8.9 years after 3rd-line ASCT) and esophageal cancer (5.2 years after 3rd-line ASCT). Two further patients with second invasive malignancies are alive following surgical tumor removal without further specific treatment: one patient with renal cancer and later occurring non-small cell lung cancer, one patient with prostate cancer.
Discussion
Our retrospective analysis demonstrates that sustained long-term remissions can be achieved in patients with peripheral T-cell-lymphomas following ASCT not only in 1st treatment line, but also in 2nd or 3rd line. It also indicates that patients with refractory disease at ASCT or residual disease after ASCT may benefit from radiotherapy. Our results of patients with 2nd-line ASCT (10-year OS and PFS 59% and 50%, respectively) are in a certain contrast with the prevailing view. Only a few publications address the effectivity of autologous stem cell transplantation in 2nd-line therapy, partly without differentiation between autologous and allogeneic stem cell transplantation. Mak et al. observed a low 3-year OS (7%) after conventional therapy without stem cell transplantation [11]. Stem cell transplantation improved the 3-year OS to 34% [15]. An additional analysis reported even higher 3-year OS (59%) and PFS (47%) following ASCT [20].
In the case of refractory disease after first salvage therapy our patient cohort usually was treated by a second salvage regimen and subsequently with ASCT in 3rd treatment line which is worthy of discussion. For a part of these patients, we planned a consolidation with allogeneic transplantation if feasible, given the promising results for example of Corradini et al. (n=17, 3-year OS and PFS 81% and 64%, respectively) and Dodero et al. (n=52, 5-year OS and PFS 50 and 40%, respectively) [42, 43]. However, no one of five allogeneically transplanted patients in our patient cohort had a sustained remission following ASCT and subsequent allogeneic transplantation. On the other hand, our results with 3rd-line ASCT and partial subsequent RT indicate a 5-year OS and PFS of 30 % and 30%, respectively, and some long-term remissions. They offer an alternative if other possible treatments are not suitable. Patients with the poorest prognosis due to a high-risk IPI at start of induction therapy did rarely proceed to HDT and ASCT in our institution and indicate a substantial unmet medical need. Probably exceptionally, we observed one patient with a high-risk IPI who developed a sustained complete remission following 1st-line ASCT.
Our data show that irradiation after ASCT is probably a favourable prognostic factor for PFS (p=0.024), although only 7 of 48 patients (15%) received this kind of treatment after ASCT in our cohort. No one of these seven patients relapsed after ASCT. However, two of these seven irradiated patients died late after ASCT (5.8 and 13.7 years later, respectively) due to invasive secondary malignancies occurring outside of the irradiation area. All seven irradiated patients had unfavourable prognostic factors after ASCT (without radiotherapy) following the results of the mentioned publications. The long-lasting complete remissions in all seven patients exceed expectations. We planned irradiation shortly after ASCT before hospital discharge and started it shortly after discharge, about 6-8 weeks after ASCT, in order to avoid early lymphoma relapse. These observations supplement our experience with irradiation after ASCT in poor prognostic aggressive B-cell-lymphoma (DLBCL) [44].
In the absence of prospective randomised trials German and American guidelines mention radiotherapy in patients with peripheral T-cell lymphomas as an option following chemotherapy. The rarity and heterogeneity of T-cell lymphomas may prohibit prospective randomized trials to adequately address this issue. This emphasizes the importance of observational studies to resolve the issue. Despite its small number and large heterogeneity of patients, our study may reflect a real-life setting without exclusion of patients with a poor prognosis.
In conclusion, our data suggest that in peripheral T-cell lymphoma patients with either residual disease early after ASCT or with progressive limited lymphoma areas shortly before ASCT may benefit from a timely irradiation. Involved-field irradiation may be an option for some patients. Further investigations are required to clarify the impact of radiotherapy added to ASCT and to identify patients for alternative treatment such as allogeneic stem cell transplantation following novel agents [42, 43, 45-47].
Acknowledgements
We thank Mona Temel for contributing to the collection of data.
Funding
None.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Bernd Metzner, Jutta Welzel, Thomas H. Müller and E. Petershofen. The first draft of the manuscript was written by Bernd Metzner and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
None.
Data Availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Ethics Approval
The protocol was approved by the Medical Ethics Committee of the Carl-von-Ossietzky University Oldenburg and carried out in accordance with the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained from all individual participants included in the study.
Article Info
Article Type
Original ArticlePublication history
Received: Tue 29, Aug 2023Accepted: Thu 21, Sep 2023
Published: Sat 30, Sep 2023
Copyright
© 2023 Bernd Metzner. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2023.02.01
Author Info
Bernd Metzner Jutta Welzel Thomas H. Müller Jochen Casper Christoph Kimmich Eduard K. Petershofen Ruth Thole Andreas Voss Kay Willborn Claus Henning Köhne
Corresponding Author
Bernd MetznerDepartment of Oncology and Hematology, Klinikum Oldenburg, University Clinic, Germany
Figures & Tables
Table 1: Characteristics
of 48 patients with peripheral T-cell lymphoma after ASCT.
|
Patients,
n |
48 |
|
Age
(years, median/range) |
54
(19-77) |
|
Sex
(female/male), n (%) |
16
(33) / 32 (67) |
|
Histology: Anaplastic Large-Cell Lymphoma, 13 of whom ALK-, 6
ALK+, 2 ALK not tested Peripheral T-Cell Lymphoma
(NOS and similar) Angioimmunoblastic T-Cell
Lymphoma Enteropathy-Associated
T-Cell Lymphoma Other T-cell lymphomas, n
(%) Stage
(Ann Arbor) 1st-line ASCT: I (enteropathy-associated TL)
II
III IV IPI
at 1st-line chemotherapy: low risk
low-intermediate risk
high-intermediate risk high risk |
21
(44)
12
(25) 11
(23) 2 (4) 2 (4) 1
(4) 2 (8) 11
(44) 11
(44) 7 (28) 11
(44) 4 (16) 1 (4) |
|
Prior
treatment with anthracyclines (CHOP or CHOEP), n (%) |
48
(100) |
|
High-dose
therapy: BEAM Mega-CHOEP
TBI/Cyclophosphamide Treatment
line: 1st-line ASCT, n (%) 2nd-line
ASCT, n (%) 3rd-line
ASCT, n (%) Time
from initial diagnosis to 1st relapse in 2nd-line ASCT,
median/range (mo) |
40
(83) 6 (13) 2 (4) 25
(52) 13
(27) 10
(21) 16
(2-123) |
|
Time
from initial diagnosis to 1st relapse in 3rd-line ASCT,
median/range (mo) Refractory
to 2nd-line chemotherapy, n (%) Second
relapse, n (%) LDH:
elevated at 1st-line ASCT, n (%),
elevated at 2nd-line
ASCT, n (%), elevated at 3rd-line
ASCT, n (%), no information at 3rd-line
ASCT |
7
(1-44) 10
(21) 0 1
(4) 1
(8) 4
(40) 1
(10) |
|
Remission
status: CR/PR/SD/PD at 1st-line ASCT, n
CR/PR/SD/PD/ED/NI after 1st-line ASCT, n CR/PR/SD/PD at
2nd-line ASCT, n CR/PR/SD/PD/ED
after 2nd-line ASCT, n CR/PR/SD/PD at
3rd-line ASCT, n CR/PR/SD/PD/ED
after 3rd-line ASCT, n |
4/21/0/0 14/8/0/1/1/1 8/5/0/0 10/1/0/1/1 0/1/5/4 3/1/1/3/2 |
|
Radiotherapy
after 1st-line ASCT, n (%) Radiotherapy
after 2nd-line ASCT, n (%) Radiotherapy
after 3rd-line ASCT, n (%) Allogeneic
stem cell transplantation after 1st-line ASCT, n (%) |
3
(12) 2
(15) 2
(20) 4
(16) |
ALK:
Anaplastic Lymphoma Kinase; TL: T-cell Lymphoma; NOS: Not Otherwise Specified;
IPI: International Prognostic Index; BEAM: High-dose BCNU, Etoposide,
Cytarabine, Melphalan; Mega-CHOEP: High-dose Cyclophosphamide, Doxorubicin,
Vincristine, Etoposide, Prednisone; TBI: Total Body Irradiation; mo: Months;
LDH: Lactic Dehydrogenase; CR: Complete Remission; PR: Partial Remission; SD:
Stable Disease; PD: Progressive Disease; ED: Early Death; NI: No Information.
Table 2: Characteristics
of 21 patients with peripheral T-cell lymphoma and long-term remission after ASCT.
|
Patients,
n |
21 |
|
Age
(years, median/range) |
54
(19-73) |
|
Sex
(female/male), n (%) |
8
(38) / 13 (62) |
|
Histology: Anaplastic Large-Cell Lymphoma, 5 of whom ALK-, 5
ALK+, 2 ALK not tested, n (%) Peripheral T-Cell Lymphoma
(NOS and similar), n (%) Angioimmunoblastic T-Cell
Lymphoma, n (%) Enteropathy-Associated
T-Cell Lymphoma, n (%) Stage
(Ann Arbor) 1st-line ASCT: I (enteropathy-associated TL), n (%)
II, n (%) III, n (%)
IV, n (%) IPI
at 1st-line chemotherapy: low risk, n (%)
low-intermediate risk, n (%) high-intermediate risk,
n (%)
high risk, n (%) |
12
(57) 4(17) 4
(17) 1
(5) 1
(5) 3
(14) 5
(24) 2
(10) 7
(33) 2(10) 1 (5) 1 (5) |
|
Prior
treatment with anthracyclines (CHOP or CHOEP), n (%) |
21
(100) |
|
High-dose
therapy: BEAM Mega-CHOEP
TBI/Cyclophosphamide Treatment
line: 1st-line ASCT, n (%) 2nd-line
ASCT, n (%) 3rd-line
ASCT, n (%) Time
from initial diagnosis to 1st relapse in 2nd-line ASCT,
median/range (mo) |
20
(95) 0 1 (5) 11
(52) 7
(33) 3
(14) 25
(4-123) |
|
Time
from initial diagnosis to 1st relapse in 3rd-line ASCT,
median/range (mo) Refractory
to 2nd-line chemotherapy, n (%) Second
relapse, n (%) LDH:
elevated at 1st-line ASCT, n (%),
elevated at 2nd-line
ASCT, n (%), elevated at 3rd-line
ASCT, n (%), |
5
(1-9) 3
(14) 0 1
(9) 1
(14) 0 |
|
Remission
status: CR/PR/SD/PD at 1st-line ASCT, n CR/PR/SD/PD
after 1st-line ASCT, n CR/PR/SD/PD at
2nd-line ASCT, n CR/PR/SD/PD/ED
after 2nd-line ASCT, n CR/PR/SD/PD at
3rd-line ASCT, n
CR/PR/SD/PD/ED/NI after 3rd-line ASCT, n |
2/9/0/0 3/8/0/0 5/2/0/0 7/0/0/0 0/0/1/2 2/1/0/0 |
|
Radiotherapy
after 1st-line ASCT, n (%) Radiotherapy
after 2nd-line ASCT, n (%) Radiotherapy
after 3rd-line ASCT, n (%) Allogeneic
stem cell transplantation |
3
(27) 2
(29) 2
(67) 0 |
ALK:
Anaplastic Lymphoma Kinase; TL: T-cell Lymphoma; NOS: Not Otherwise Specified;
IPI: International Prognostic Index; BEAM: High-dose BCNU, Etoposide,
Cytarabine, Melphalan; Mega-CHOEP: High-dose Cyclophosphamide, Doxorubicin,
Vincristine, Etoposide, Prednisone; TBI: Total Body Irradiation; mo: Months;
LDH: Lactic Dehydrogenase; CR: Complete Remission; PR: Partial Remission; SD:
Stable Disease; PD: Progressive Disease; ED: Early Death; NI: No Information.


References
1.
d’Amore
F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H et al. (2012) Up-Front
Autologous Stem-Cell Transplantation in Peripheral T-Cell Lymphoma NLG-T-01. J Clin Oncol 30: 3093-3099. [Crossref]
2.
Feyler S, Prince HM, Pearce R, Towlson K, Nivison Smith I et
al. (2007) The role of high-dose therapy and stem cell rescue in the management
of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow
Transplant 40: 443-450. [Crossref]
3.
Zhai Y, Wang J, Jiang Y, Jiang Y, Wu W et al. (2022) The
efficiency of autologous stem cell transplantation as the first-line treatment
for nodal peripheral T-cell lymphoma: results of a systematic review and
meta-analysis. Expert Rev Hematol 15: 265-272. [Crossref]
4.
Advani RH, Skrypets T, Civallero M, Spinner MA, Manni M et
al. (2021) Outcomes and prognostic factors in angioimmunoblastic T-cell
lymphoma: final report from the international T-cell Project. Blood 138:
213-220. [Crossref]
5.
Al Mansour Z, Li H, Cook JR, Constine LS, Couban S et al.
(2019) Autologous Transplantation as Consolidation for High-Risk Aggressive
T-Cell Non-Hodgkin's Lymphoma: A SWOG 9704 Intergroup Trial Subgroup Analysis. Leuk
Lymphoma 60: 1934-1941. [Crossref]
6.
Fossard G, Broussais F, Coelho I, Bailly S, Nicolas
Virelizier E et al. (2018) Role of up-front autologous stem-cell
transplantation in peripheral T-cell lymphoma for patients in response after
induction: an analysis of patients from LYSA centers. Ann Oncol 29:
715-723. [Crossref]
7.
Boo Y.L. Koh LP (2021) Hematopoietic Stem Cell
Transplantation in T Cell and Natural Killer Cell Lymphomas: Update on Recent
Advances. Transplant Cell Ther 27: 571-588. [Crossref]
8.
Schmitz N, Truemper L, Bouabdallah K, Ziepert M, Leclerc M et
al. (2021) A randomized phase 3 trial of autologous vs allogeneic transplantation
as part of first-line therapy in poor-risk peripheral T-NHL. Blood 137:
2646-2656. [Crossref]
9.
Schmitz
N, Trümper L, Ziepert M, Nickelsen M, Ho AD et al. (2010) Treatment
and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients
with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin
Lymphoma Study Group. Blood 116: 3418-3425. [Crossref]
10. Wilhelm M, Smetak M, Reimer P, Geissinger E, Ruediger T et
al. (2016) First-line therapy of peripheral T-cell lymphoma: extension
and long-term follow-up of a study investigating the role of autologous stem
cell transplantation. Blood Cancer J 6: e452-e457. [Crossref]
11. Mak
V, Hamm J, Chhanabhai M, Shenkier T, Klasa R et al. (2013) Survival of Patients
with Peripheral T-Cell Lymphoma After First Relapse or Progression: Spectrum of
Disease and Rare Long-Term Survivors. J Clin Oncol 31: 1970-1976. [Crossref]
12. Kim
MK, Kim S, Lee SS, Sym SJ, Lee DH et al. (2004) High-dose chemotherapy and
autologous stem cell transplantation for peripheral T-cell lymphoma: complete
response at transplant predicts survival. Ann Hematol 86: 435-442. [Crossref]
13. Kitahara H, Maruyama D, Maeshima AM, Makita S, Miyamoto KI
et al. (2017) Prognosis of patients with peripheral T cell lymphoma who
achieve complete response after CHOP/CHOP-like chemotherapy without autologous
stem cell transplantation as an initial treatment. Ann Hematol 96:
411-420. [Crossref]
14. Reimer P, Rüdiger T, Geissinger E, Weissinger F, Nerl C et
al. (2009) Autologous Stem-Cell Transplantation As First-Line Therapy in
Peripheral T-Cell Lymphomas: Results of a Prospective Multicenter Study. J
Clin Oncol 27: 106-113. [Crossref]
15. Zhang
JY, Briski R, Devata S, Kaminski MS, Phillips TJ et al. (2018) Survival
Following Salvage Therapy for Primary Refractory Peripheral T-Cell Lymphomas
(PTCL). Am J Hematol 93: 394-400. [Crossref]
16.
Hapgood G, Savage KJ (2015) The biology and management of
systemic anaplastic large cell lymphoma. Blood 126:
17-25. [Crossref]
17. Glass
B, Kloess M, Bentz M, Schlimok G, Berdel WE et al. Dose-escalated CHOP plus
etoposide (MegaCHOEP) followed by repeated stem cell transplantation for
primary treatment of aggressive high-risk Non-Hodgkin’s Lymphoma (aNHL). Blood 107:
3058-3064. [Crossref]
18. Schmitz N, Kloess M, Reiser M, Berdel
WE, Metzner B et al. (2006) Four
versus six courses of a dose-escalated cyclophosphamide, doxorubicin,
vincristine, and prednisone (CHOP) regimen plus etoposide (megaCHOEP) and
autologous stem cell transplantation: early dose intensity is crucial in
treating younger patients with poor prognosis aggressive lymphoma. Cancer
106: 136-145. [Crossref]
19. Nickelsen M, Ziepert M, Zeynalova S, Glass B, Metzner B et
al. (2009) High-dose CHOP plus etoposide (MegaCHOEP) in T-cell lymphoma:
a comparative analysis of patients treated within trials of the German
High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Ann Oncol 20:
1977-1984. [Crossref]
20. Smith
SM, Burns LJ, van Besien K, Lerademacher J, He W et al. (2013) Hematopoietic
Cell Transplantation for Systemic Mature T-Cell Non-Hodgkin Lymphoma. J Clin Oncol 31: 3100-3109. [Crossref]
21. Petrich AM, Helenowski IB, Bryan LJ, Rozell SA, Galamaga R
et al. (2015) Factors predicting survival in peripheral T-cell lymphoma in
the USA: a population-based analysis of 8802 patients in the modern era. Brit
J Haematol 168: 708-718. [Crossref]
22. Sibon
D, Fournier M, Brière J, Lamant L, Haioun C et al. (2012) Long-Term Outcome of Adults
With Systemic Anaplastic Large-Cell Lymphoma Treated Within the Groupe d'Etude
des Lymphomes de l'Adulte Trials. J Clin Oncol 30: 3939-3946. [Crossref]
23. Park
SI, Horwitz SM, Foss FM, Pinter Brown LC, Carson KR et al. (2019) The Role of
Autologous Stem Cell Transplantation in Patients With Nodal Peripheral T-Cell
Lymphomas in First Complete Remission: Report From COMPLETE, a Prospective,
Multicenter Cohort Study. Cancer 125: 1507-1517. [Crossref]
24. Yang DH, Kim WS, Kim SJ, Bae SH, Kim SH et al. (2009) Prognostic
Factors and Clinical Outcomes of High-Dose Chemotherapy followed by Autologous
Stem Cell Transplantation in Patients with Peripheral T Cell Lymphoma,
Unspecified: Complete Remission at Transplantation and the Prognostic Index of
Peripheral T Cell Lymphoma Are the Major Factors Predictive of Outcome. Biol
Blood Marrow Transplant 15: 118-125. [Crossref]
25. Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, Reimer P
et al. (2017) Clinical Practice Recommendations on Indication and Timing of
Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An
International Collaborative Effort on Behalf of the Guidelines Committee of the
American Society for Blood and Marrow Transplantation. Biol Blood Marrow
Transplant 23: 1826-1838. [Crossref]
26. Horwitz
S, O´Connor OA, Pro B, Illidge T, Fanale M et al. (2019) Brentuximab vedotin
with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a
global, double-blind, randomised, phase 3 trial. Lancet 393: 229-240. [Crossref]
27. Laribi
K, Alani M, Truong C, de Materre AB (2018) Recent Advances in the Treatment of
Peripheral T‐Cell
Lymphoma. Oncologist 23: 1039-1053. [Crossref]
28. Maurer
MJ, Ellin F, Srour L, Jerkeman M, Bennani et al. (2017) International
Assessment of Event-Free Survival at 24 Months and Subsequent Survival in
Peripheral T-Cell Lymphoma. J Clin Oncol 35: 4019-4026. [Crossref]
29. Lunning
MA, Vose JM (2017) Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma.
Blood 129: 1095-1102. [Crossref]
30. Ng
AK, Yahalom J, Goda JS, Constine LS, Pinnix CC et al. (2018) Role of Radiation
Therapy in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma:
Guidelines from the International Lymphoma Radiation Oncology Group. Int J
Radiat Oncol Biol Phys 100: 652-669. [Crossref]
31. Dhakal
S, Bates JE, Casulo C, Friedberg JW, Becker MW et al. (2016) Patterns and
Timing of Failure for Diffuse Large B-Cell Lymphoma After Initial Therapy in a
Cohort Who Underwent Autologous Bone Marrow Transplantation for Relapse. Int
J Radiat Oncol Biol Phys 96: 372-378. [Crossref]
32. Horwitz
SM, Ansell S, Ai WZ, Barnes J, Barta SK et al. (2022) T-Cell Lymphomas, Version
2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc
Netw 20: 285-308. [Crossref]
33. Melchers RC, Willemze R, Daniels LA, Neelis KJ, Bellenk MW
et al. (2017) Recommendations for the Optimal Radiation Dose in Patients
With Primary Cutaneous Anaplastic Large Cell Lymphoma: A Report of the Dutch
Cutaneous Lymphoma Group. Int J Radiat Oncol Biol Phys 99: 1279-1285. [Crossref]
34. Shikama
N (2019) Local radiation for cutaneous T-cell lymphoma other than mycosis
fungoides and Sézary syndrome. Chin Clin Oncol 8: 8. [Crossref]
35. Deng T, Zhang C, Zhang X, WuS, Xu Y et al. (2014) Treatment
Outcome of Radiotherapy Alone versus Radiochemotherapy in IE/IIE Extranodal
Nasal-Type Natural Killer/T Cell Lymphoma: A Meta-Analysis. PLoS One 9:
e106577. [Crossref]
36. Jiang L, Li SJ, Jiang YM, Long JX, Wang RS et al. (2014) The
significance of combining radiotherapy with chemotherapy for early stage
extranodal natural killer/T-cell lymphoma, nasal type: a systematic review and
meta-analysis. Leuk Lymphoma 55: 1038-1048. [Crossref]
37. Zang J, Li C, Luo SQ, Wang JH, Xu Man et al. (2015) Early
radiotherapy has an essential role for improving survival in patients with
stage I-II nasal-type of NK/T cell lymphoma treated with
L-asparaginasecontaining chemotherapy--a single institution experience. Ann
Hematol 94: 583-591. [Crossref]
38. Zhou
YM, Liu X, Yang Y, Wang SL, Fang H et al. Effects of gross tumor volume and
radiation dose on survival and locoregional recurrence in early-stage
extranodal NK/T-cell lymphoma treated with intensity-modulated radiation
therapy. J Cancer Research Clin Oncol 149: 5219-5230. [Crossref]
39. Wu T, Yang Y, Zhu SY, Shi M, Su H et al. (2018) Risk-adapted
survival benefit of IMRT in early-stage NKTCL: a multicenter study from the
China Lymphoma Collaborative Group. Blood Adv 2: 2369-2377. [Crossref]
40. de
Pádua Covas Lage LA, Machado PPF, Reichert CO, Miranda E, Culler HF et al.
(2022) High‑dose
extended‑field
radiotherapy plus chemotherapy improved survival in extranodal NK/T‑cell lymphoma in a real‑life setting: results from
the multicenter T‑Cell
Brazil Project. Sci Rep 12: 20557. [Crossref]
41. Patel
AM, West L, Atluri PS, Yi SF, Rizvi S et al. (2023) Optimizing Palliative Focal
Radiation Therapy Dose in Cutaneous T-Cell Lymphoma: How Low Can You Go? Pract
Radiat Oncol 13: e192-e199. [Crossref]
42.
Corradini
P, Dodero A, Zallio F, Caracciolo D, Bregni M et al. (2004)
Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's
lymphomas after reduced-intensity conditioning followed by allogeneic transplantation
of hematopoietic cells. J Clin Oncol 22: 2172-2176. [Crossref]
43.
Dodero
A, Spina F, Narni F, Patriarca F, Cavattoni I et al. (2012) Allogeneic
transplantation following a reduced-intensity conditioning regimen in
relapsed/refractory peripheral T-cell lymphomas: long-term remissions and
response to donor lymphocyte infusions support the role of a
graft-versus-lymphoma effect. Leukemia 26: 520-526. [Crossref]
44. Metzner
B, Welzel J, Müller TH, Casper J, Kimmich C et al. (2022) Long-term remissions
in patients with early relapse of diffuse large B-cell lymphoma following high-dose
chemotherapy, autologous stem cell transplantation, and radiotherapy of
residual disease. Strahlenther Onkol 198: 39-46. [Crossref]
45.
Le
Gouill S, Milpied N, Buzyn A, De Latour RP, Vernant JP et al. (2008)
Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study
by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J
Clin Oncol 26: 2264-2271. [Crossref]
46. Mamez AC, Dupont A, Blaise D, Chevallier P, Forcade E et al. (2020) Allogeneic stem cell transplantation for peripheral T cell lymphomas: a retrospective study in 285 patients from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). J Hematol Oncol 13: 56. [Crossref]
47. Mehta-Shah N, Kommalapati A, Teja S, Cashen AF, Dahi PB, Sauter CS et al. (2020) Successful Treatment of Mature T-Cell Lymphoma with Allogeneic Stem Cell Transplantation: The Largest Multicenter Retrospective Analysis. Blood 136: 35-36.
