Journals
Long-term Follow-up in a Patient with Segmentectomy for Lung Cancer Developed in the Segment with Displaced Left B1+2 Bronchus
A B S T R A C T
A 62-year-old woman was admitted with a 20 mm diameter tumor in the left upper lobe of the lung. Preoperative computed tomography (CT) revealed a displaced anomalous B1+2 arising from the left main bronchus. In addition, an accessory fissure was detected between the apicoposterior (S1+2) and anterior (S3) segments. As the lesion was entirely contained in the S1+2, we performed video-assisted thoracic surgical S1+2 segmentectomy with systematic lymph node dissection. During the operation, we easily detected and successfully divided the displaced B1+2 located behind the left main pulmonary artery. The pathological diagnosis was invasive adenocarcinoma with T1bN0M0 (TNM 8th edition). Histologically, neither lymphatic invasion nor vascular invasion was detected in the invasive area of the tumor. If regional lymph node dissection with appropriate intraoperative histologic examination by frozen section could be made, segmentectomy may be an acceptable, optional procedure with curative intent for cancer patients with such an anomaly.
K E Y W O R D S
Displaced left B1+2 bronchus, segmentectomy, lung cancer
Introduction
A comprehensive preoperative evaluation of the lung anatomy is essential for preventing unexpected intraoperative complications due to bronchial and vessel anomalies. According to the review of bronchographies of 13,222 cases, there were only two cases (0.015%) with the displaced left B1+2 [1]. Moreover, lung cancer originating from the apicoposterior segment (S1+2) with a displaced left B1+2 is extremely rare, and there is no standard surgical procedure for such a combination. We herein report the successful treatment of a patient with lung cancer combined with a displaced left B1+2 based on precise preoperative anatomic information and long-term postoperative follow-up.
Case Report
A 62-year-old woman had been referred to another hospital because of an abnormal shadow on screening chest radiography. She had no symptoms. Serum tumor markers were not elevated. Computed tomography (CT) revealed a subsolid nodule with a maximal diameter of 20 mm in the left upper lobe (Figure 1). The displaced B1+2 ascending behind the left main pulmonary artery and an accessory fissure were also found. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) revealed a weak uptake for the tumor (SUVmax=2.5). Neither lymph node metastasis nor distant metastasis was detected. Although the histologic diagnosis was not confirmed, she was transferred to Yao Municipal Hospital for surgical treatment.
Preoperative analysis of CT findings was performed using three-dimensional reconstruction images. As a result, a left B1+2 arose from the left main bronchus and ascended behind the left main pulmonary artery (Figure 2). In addition, an accessory fissure was detected between the apicoposterior segment (S1+2) and anterior segment (S3) with hypoplastic bronchus (B3) arose from the left B4 (Figure 2B). This patient for whom surgical procedure was planned provided written informed consent after fully talking over the risks and benefits with surgeons.
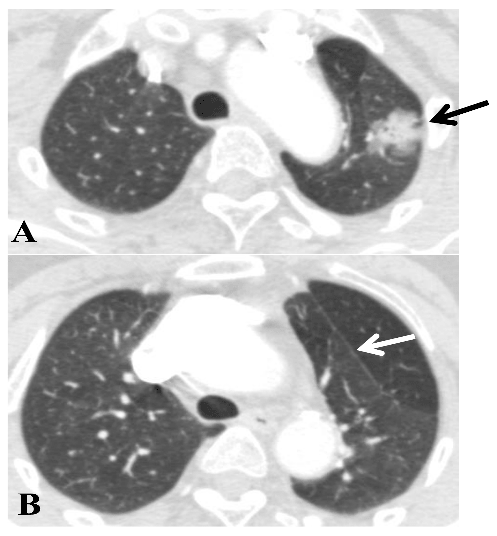
A: tumor in the S1+2 (black arrow).
B: white arrow showing the accessory fissure between S1+2 and S3.
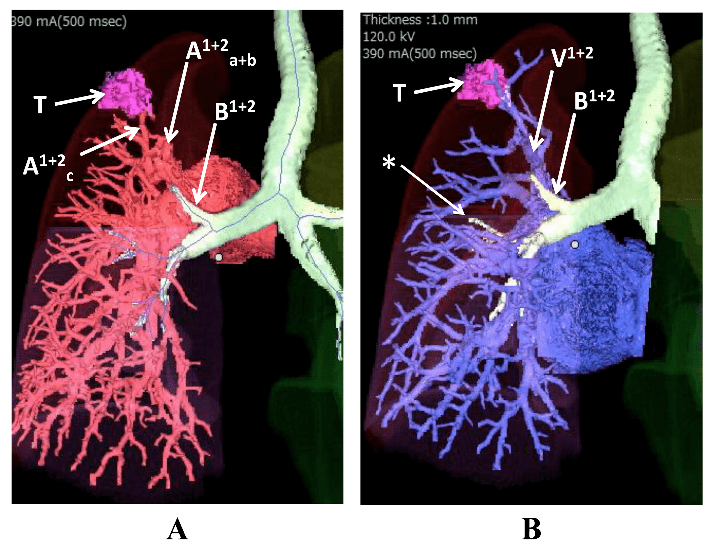
A: displaced B1+2 ascending behind the left main pulmonary artery.
B: relationship between B1+2 and V1+2. T: tumor. *: hypoplastic B3.
Video-assisted thoracic surgical S1+2 segmentectomy with lymph node dissection was performed as follows: The S1+2 and the S3 was separated by an accessory fissure. Among the major branches of the superior pulmonary vein, the apicoposterior vein (V1+2) was isolated, ligated, and divided. Then, the displaced B1+2 ascending behind V1+2 was identified. For a safer approach, we divided the apicoposterior artery (A1+2) directory arose from the left main pulmonary artery (PA) in the same way. Then, the displaced B1+2 was dissected free from its surrounding lymphatic tissue. We clamped this bronchus and performed an inflation test to confirm that the tumor was entirely contained within the S1+2 segment. After stapling of the B1+2, we divided incomplete interlobar fissure (S1+2/S6) with the stapler, and the specimen was removed from the surgical field. The intraoperative lavage cytology of the resection margin (S1+2/S6) was negative. We dissected lymph nodes along with the lymphatic pathway from peribronchial lymph node of B1+2 regarding as a part of hilum (#12 and #10) to subaorta (#5). Intraoperative frozen section histology of #12 and #5 lymph nodes was negative. Bronchial stump was negative at permanent section histology. The pathological diagnosis was T1bN0M0 invasive adenocarcinoma with a 19.5 x 11mm invasive cancer area in the 20 x 12 mm tumor. There was no lymphatic or vascular invasion. Her tumor harbored L878R mutation in EGFR.
The postoperative course was uneventful, and the patient was discharged from the hospital 11 days after surgery. She had no postoperative adjuvant therapy and is now well without recurrence for 65 months after surgery. A minimal change in pulmonary function testing before and at 5 years after surgery was observed, with a forced vital capacity (FVC) of 2.33 L and 2.00 L, and a forced expiratory volume in 1 second (FEV1.0) of 1.82 L and1.67 L, respectively.
Discussion
Three major hypotheses concerning the pathogenesis of anomalous bronchial development have been formulated to explain anomalous bronchi: the reduction, migration, and selection theories [2]. The variations in bronchial bifurcation observed in 30 consecutive asymptomatic and healthy patients were reported by Ghaye et al. [2]. Most variations are seen in the right upper lobe as the typical trifurcation of segmental bronchi. On the contrary, a displaced left B1+2, the so-called left tracheal bronchus, is rare.
Our report highlights two points. Firstly, precise preoperative anatomic information on the bronchus and lung vessels is essential to prevent unexpected intraoperative complications. According to our search of the literature , only 7 cases of lung cancer associated with a displaced B1+2 including the present case have been reported (Table 1) [3-8]. In 6 of the 7 cases, the displaced B1+2 was located behind the left pulmonary artery. In 2 previously reported cases, the displaced B1+2 was unexpectedly divided by a stapler at the time of interlobar fissure completion. In both cases, the displaced B1+2 could be identified preoperatively, but the comprehensive positional relationship between the displaced B1+2 and left main pulmonary artery was nor clarified. In our case, the use of multiplanar reconstruction CT with a 3-dimensional volume analyzer made it possible to clearly depict the displaced B1+2 that originated behind the left main pulmonary artery.
Secondly, there has been no report concerning the oncological outcomes after segmentectomy for patients with invasive adenocarcinoma in the isolated left S1+2. Sienel et al. advised against the segmentectomy of stage 1A non-small cell lung cancer (NSCLC) in the S1-3 region because of a higher local recurrence rate (23%) [9]. On the other hand, Soukiasian et al. reported that video-assisted thoracoscopic trisegmentectomy is associated with a similar survival rate to lobectomy for stage IA and IB lung cancer. According to a recent report by Altorki et al., sublobar resection and lobectomy are associated with equivalent survival rates for patients with clinical stage IA NSCLC showing solid nodules [10, 11]. Lymphatic invasion is associated with a significantly poorer overall survival in pathological node-negative T1a NSCLC (TNM 7th edition) [12]. When we consider the lymph’s centripetal transport and pathway along with this unique anomalous bronchus, anatomical S1+2 segmentectomy with regional lymph node dissection seems to be an acceptable technique with curative intent for the present case. To date, segmentectomy has been performed for only two patients with adenocarcinoma with the same anomaly (Table 1). One of them underwent S1+2 segmentectomy because of advanced age [4]. Another underwent tri-segmentectomy of left S1+2 and S6 because of severe incomplete lobulation [8]. However, according to our knowledge, there has been no report of a case with long-term follow up longer than 5 years except our report.
We previously reported our rationale of surgical treatment for c-T1aN0M0 (TNM 7th edition) lesions. Basically, we employ standard lobectomy in patients with 16-20mm diameter and < 50% GGO lesion on HRCT [13]. However, this patient is an exceptional case with bronchial anomaly and accessory fissure. As this patient had incomplete interlobar fissure between S1+2 and S6 segment, we carefully made the interlobar line with the stapler and confirmed to be cancer-free at intraoperative resection margin cytology [14].
In conclusion, we successfully performed left S1+2 segmentectomy for a patient with stage 1A invasive adenocarcinoma entirely contained in the S1+2 with a displaced left B1+2 and accessory fissure between S1+2 and S3. Personalized surgery should be planned based on precise information from preoperative multiplanar reconstruction CT imaging with a 3-dimensional volume analyzer when a trachea-bronchial anomaly is suspected on a routine CT scan.
Table 1: Reported cases of lung cancer associated with displaced B1+2
|
|
||||||||
|
Author (Year) |
Age/Sex |
Procedure |
Preoperative |
Position of |
|
Histology |
p-stageT1bN0 |
Survival (Months) |
|
Motohashi (1995) |
52/F |
LP |
+ |
ND |
|
Squamous |
T2N1M1 |
10 |
|
Shimamoto (2008) |
81/F |
S1+2 |
+ |
Behind LMPA |
+ |
Adenoca. |
T1bN0M0 |
10 |
|
Tsukioka (2011) |
62/F |
LUL |
+ |
Behind LMPA |
+ |
Adenoca. |
T1bN0M0 |
ND |
|
Asakura (2013) |
52/M |
LUL |
+ |
Behind LMPA |
|
Adenoca. |
T2aN0M0 |
12 |
|
Ikuta (2013) |
83/M |
LUL |
+ |
Behind LMPA |
|
Squamous |
T2aN0M0 |
ND |
|
Nakamura (2017) |
66/F |
S1+2+S6 |
|
Behind LMPA |
|
Adenoca |
T2aN0M0 |
ND |
|
Yamato(2018) |
62/F |
S1+2 |
+ |
Behind LMPA |
|
Adenoca. |
T1aN0M0 |
65 |
|
Br: bronchus; LP: left pneumonectomy; ND: not described; S1+2: S1+2 segmentectomy; S1+2+S6: S1+2+S6 trisegmentectomy; |
||||||||
|
LMPA: left main pulmonary artery; LUL: left upper lobectomy |
||||||||
Consent
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Conflict of interest
All authors declare that they have no conflict of interest.
Article Info
Article Type
Case ReportArticle History
Received 17 November, 2018Accepted 20 December, 2018
Published 28 December, 2018
Copyright
© 2018 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.10.31487/j.SCR.2018.03.022
Author Info
Corresponding author
Ken KodamaDepartment of Thoracic Surgery, Yao Municipal Hospital, 1-3-1 Ryuge-cho, Yao City, Osaka 581-0069, Japan.
Figures & Tables

A: tumor in the S1+2 (black arrow).
B: white arrow showing the accessory fissure between S1+2 and S3.

A: displaced B1+2 ascending behind the left main pulmonary artery.
B: relationship between B1+2 and V1+2. T: tumor. *: hypoplastic B3.
Table 1: Reported cases of lung cancer associated with displaced B1+2
|
|
||||||||
|
Author (Year) |
Age/Sex |
Procedure |
Preoperative |
Position of |
|
Histology |
p-stageT1bN0 |
Survival (Months) |
|
Motohashi (1995) |
52/F |
LP |
+ |
ND |
|
Squamous |
T2N1M1 |
10 |
|
Shimamoto (2008) |
81/F |
S1+2 |
+ |
Behind LMPA |
+ |
Adenoca. |
T1bN0M0 |
10 |
|
Tsukioka (2011) |
62/F |
LUL |
+ |
Behind LMPA |
+ |
Adenoca. |
T1bN0M0 |
ND |
|
Asakura (2013) |
52/M |
LUL |
+ |
Behind LMPA |
|
Adenoca. |
T2aN0M0 |
12 |
|
Ikuta (2013) |
83/M |
LUL |
+ |
Behind LMPA |
|
Squamous |
T2aN0M0 |
ND |
|
Nakamura (2017) |
66/F |
S1+2+S6 |
|
Behind LMPA |
|
Adenoca |
T2aN0M0 |
ND |
|
Yamato(2018) |
62/F |
S1+2 |
+ |
Behind LMPA |
|
Adenoca. |
T1aN0M0 |
65 |
|
Br: bronchus; LP: left pneumonectomy; ND: not described; S1+2: S1+2 segmentectomy; S1+2+S6: S1+2+S6 trisegmentectomy; |
||||||||
|
LMPA: left main pulmonary artery; LUL: left upper lobectomy |
||||||||
References
1. Ohta S, Saito Y, Usuda K, Kanma K, Sagawa M, et al. (1986) Tracheobronchial anomalies: report of 71 cases. JJSB 8: 122-130.
2. Ghaye B, Szapiro D, Franchamps FM, Dondelinger RF (2001) Congenital bronchial abnormalities revisited. Radio Graphics 21: 105-119. [Crossref]
3. Motohashi S, Yamaguchi Y, Takeda T, Aoyagi H, Ohtsuka T, et al. (1995) A case of lung squamous cell carcinoma in anomalously situated subsegmental bronchus of left upper lobe; a case report. The J Japanese Associat Chest Surg 9: 181-186.
4. Shimamoto A, Takao M, Kodama H, Murashima S, Shomura S, et al. (2008) A case of left apicoposterior segmentectomy for lung cancer occurring in a displaced anomalous bronchus. The J Japan Society for Respiratory Endoscopy 30: 210-214.
5. Tsukioka T, Yamamoto R, Takahama M, Nakajima T, Tada H (2011) A case of lung cancer arising from abnormal bronch. The J Japanese Associat Chest Surg 25: 460-464.
6. Asakura K, Imanishi N, Matsuoka T, Nagai S, Matsuoka K, et al. (2013) Video-assisted thoracic surgery lobectomy for lung cancer with displaced B1+2. Ann Thorac Cardiovasc Surg 20: 486-489. [Crossref]
7. Ikuta Y, Tamura K, Sakamoto A, Hidaka K. (2013) Lung cancer in the left upper lobe with a displaced anomalous left B1+2bronchus accompanied by an anomalous V1+2pulmonary vein: A surgical case. The J Japanese Associat Chest Surg 27: 729-733.
8. Nakamura A, Watanabe S, Watanabe Y, Asakura K, Nakagawa K (2017) En bloc upper and lower lobe trisegmentectomy facilitated by displaced segmental airway. Ann Thorac Surg 104: 447-449. [Crossref]
9. Sienel W, Stremmel C, Kirschbaum A, Hinterberger L, Stoelben E, et al. (2007) Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment location and width of resection margins – implications for patient selection for segmentectomy. Eur J Cardio-thoracic Surg 31: 522-528. [Crossref]
10. Soukiasian HJ, Hong E, McKenna J (2012) Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg 144: 23-26. [Crossref]
9. Altorki NK, Yip R, Hanaoka T, Bauer T, Aye R, et al. (2014) Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 147: 754-764. [Crossref]
10. Nentwich MF, Bohn BA, Uzunoglu FG, Reeh M, Quaas A, et al. (2013) Lymphatic invasion predicts survival in patients with early node-negative non-small cell lung cancer. J Thorac Cardiovasc Surg 146: 781-787. [Crossref]
11. Kodama K, Higashiyama M, Okami J, Tokunaga T, Imamura F, et al. (2016) Oncologic outcomes of segmentectomy versus lobectomy for clinical T1a N0 M0 non-small cell lung cancer. Ann Thorac Surg 101: 504-511. [Crossref]
12. Higashiyama M, Kodama K, Takami K, Higaki N, Nakayama T, et al. (2003) Intraoperative lavage cytologic analysis of surgical margins in patients undergoing limited surgery for lung cancer. J Thorac Cardiovasc Surg 125: 101-107. [Crossref]
