Idh-1 Status and Venous Thromboembolism in Glioma Patients
A B S T R A C T
Background: Venous thromboembolic events (VTE) are common causes of morbidity and mortality in glioblastoma patients. Mutation in the isocitrate dehydrogenase 1 enzyme (IDH1) is frequent in secondary glioblastoma and results in altered metabolomics.
Objectives: This study evaluates whether IDH-1 status correlates with incidence of VTE in glioblastoma patients.
Methods: Observational study of 398 cases of patients with glioblastoma, who all underwent surgery in a regional Neurosurgical centre between April 2012 and December 2014. IDH -1 status and Tissue factor (F3) protein expression were assessed by immunohistochemistry. Deep venous thrombosis (DVT) and pulmonary embolism (PE) were diagnosed by Doppler ultrasound and pulmonary CT angiogram respectively.
Results: 336 cases were wild type (WT) IDH-1 (94.1%) and 21 cases were IDH-1 mutated (R132H) (5.9%). 51 patients had a thromboembolic event (15.3%), with all cases of VTE in WT IDH-1 tumours, a rate of 21.8% within this group. IDH-1 status had a significant correlation with VTE (p=0.033 Fisher exact test). As expected, mutant IDH was associated with prolonged patient survival (p=0.024 Log rank). The mean expression in IDH-1 wild type GBM was 7.14 and in R132h mutant GBM was 4.87 (log2 scale). This was highly statistically significant with a corrected P value of less than 0.0001.
Conclusion: A significant association exists between IDH1 status in glioblastoma patients and the risk of VTE. Patients with wild type IDH-1 appear at high risk of VTE and appropriate precautions should be considered
Keywords
Glioblastoma, Isocitrate dehydrogenase 1, venous thromboembolic events
Introduction
Glioblastoma (GBM) is the most common primary brain malignancy, accounting for 45.2% of primary brain cancers, 54% of all gliomas, and 16% of all primary brain and CNS tumors [1]. The median survival rate for GBM is 15 months even with aggressive surgical resection, chemotherapy, and radiation [2]. It was in GBM that the key link was established between cancer and isocitrate dehydrogenase (IDH) mutations [3]. The IDH1 gene, located on chromosome 2, encodes for the isocitrate dehydrogenase enzyme that plays a role in the tri-carboxylic acid cycle in humans. Mutation in IDH-1 is most commonly a missense mutation of arginine to histidine at the R132 position though other rarer mutations occur and IDH2 can also be affected [4]. The postulated oncogenetic role of IDH mutations as a direct driver is supported by 2 mechanisms. Firstly, somatic mosaicisim for IDH-1 and 2 causes the enchondromatosis syndromes, Ollier’s disease [5]. Secondly, the introduction of mutant IDH into normal cells causes increased proliferation, increased colony formation, and inability to differentiate [6, 7]. Despite being likely to be key to the oncogenesis of tumours, IDH mutations in glioblastoma are associated with improved prognosis, with IDH mutant GBM carrying a better prognosis than grade 2 astrocytoma with wild type IDH [8, 9].
The poor prognosis of GBM has been attributed to a number of factors, but it is clear that medical comorbidities have a negative effect on survival, possibly by limiting patients’ medical fitness for adjuvant therapy. One of the most common comorbidities in patients with cancer is venous thromboembolism (VTE). Patients with malignant brain tumours are even more prone to develop VTE than those with other cancers [10, 11]. The incidence of thromboembolic events in patients with high grade gliomas is high, reaching up to 30% [12]. VTE risk reaches its peak in perioperative and early postoperative periods [12, 13]. Several risk factors have been studied to explain the development of thrombosis namely: prolonged immobility, old age, limb paresis, etc. However, the exact mechanism of the specifically elevated VTE among patients with GBM is yet to be understood.
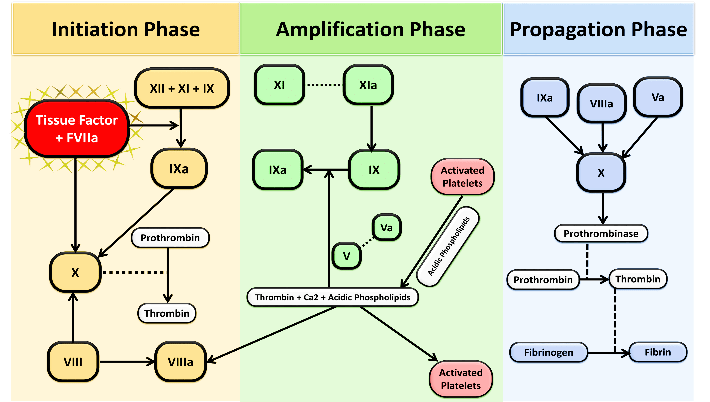
VTE seems to occur as a result of errors happening within the coagulation cascade. The new model of coagulation cascade is divided into distinct phases: (1 initiation, (2) amplification, and (3) propagation [14, 15]. Recent advances have implicated Tissue Factor (TF) as primary initiator of the coagulation cascade in vivo. TF activates the factor VII/VIIa complex, which triggers the activation of both factor X and factor IX, which in turn lead to formation of thrombin and ultimately cross-linked fibrin (Figure 1).
Figure 1: showing the different phases of coagulation cascade, which replaced the old extrinsic and intrinsic pathways
A mounting body of evidence suggests a significant role for TF in venous thromboembolism. Increased levels of TF, both circulating and cell associated, have been reported in patients with thromboembolism [16-18]. Moreover, high titers of TF antigen were detected in human deep vein thrombi [19]. Most of the studies linking TF and VTE were on patients with malignancy recognised as being up to seven-fold more likely to develop VTE as compared to normal individuals [20, 21]. Several factors potentially link TF to such increased risk in cancer patients. Of the most important ones are hypercoagulability caused by circulating tumor-cell derived TF micro-particles and possibly the abnormal expression of tissue factor in a variety of cancers [21]. Various laboratory and clinical factors were studied to explain the correlation between glioblastoma and increased incidence of VTE. Of these, IDH-1 status has been suggested to be the most influential. An American group looked into such correlation and found that VTE occurred in 26-30 % of patients with wild-type IDH1 gliomas, but not in patients with mutant IDH1 gliomas (0 %) [22]. This study aims to determine if there is a strong association between IDH-1 status and the risk of VTE in GBMs within a cohort of patients undergoing surgery and whether the tissue factor expression is associated with VTE rates, potentially identifying patients in high or low risk groups and directing stratified prophylactic approaches.
Material and Methods
This is an Observational Study in which, we retrospectively reviewed 398 patients with glioblastoma (WHO grade IV), who all underwent surgery in Regional Neurosurgical centre between April 2012 and December 2014. We included in this study all patients that had received a pathological diagnosis of glioblastoma after undergoing surgery, excluding the patients who were diagnosed solely on imaging grounds. In this study we report on instances of VTEs within 6 months after surgery regardless of whether the patients were hospitalized at the time of VTE onset. The information collected included the patient’s age at the time of surgery, details of surgery and location of the VTE if present. Data were collected for the recorded surgical resection of a GBM. The current protocol for VTE prevention in our hospital recommends all patients should receive mechanical prophylaxis in the form of compression stockings, Flowtron boots intra-operatively and prophylactic doses of low molecular weight heparin SC from 48 hours postoperatively until fully mobile. Detection and confirmation of clinically significant VTE was performed by both clinical and diagnostic means. Patients’ subjective complaints and physical examination findings (pain, swelling, erythema, extremity asymmetry) were reviewed and documented daily whilst an inpatient. Clinically suspected deep venous thrombosis in the lower extremities was confirmed by Doppler ultrasonograms and pulmonary embolism confirmed by pulmonary CT angiogram.
I Immunohistochemistry
Immunohistochemistry for IDH-1 was performed using Anti-IDH1 R132H (Hu) from Mouse (Clone: H09) (DIA-H09 Dianova). We did not assess for the rarer IDH1 and IDH2 mutations. Procoagulant thromboplastin (Tissue factor) F3 protein expression was determined by immunohistochemical staining (using Dako kit and steamer) of tissue samples. Sections at a thickness of 3-5μm were cut from routine formalin-fixed paraffin- embedded (FFPE) tumor tissue blocks. Staining against TF was performed with the monoclonal mouse anti- tissue factor antibody (Anti-Tissue Factor antibody [EPR8986] ab1517480, Abcam). Such Staining was done through a series of steps: Firstly, removal of paraffin wax and antigen retrieval followed by application of peroxidase blocking solution (100 μl), after which application of the primary antibody diluted in Dako antibody diluent (1:200), then addition of secondary antibody (3 drops). Finally, we added DAB (100 μl) solution (1:50 DAB solution in substrate buffer).
For detection of the TF expression (using OLYMPUS BX41), anti-tissue factor antibody was used in a dilution of 1:200. A pancreas sample served as positive control for TF. Procoagulant staining intensity was semi-quantitatively classified into: (-) – no expression; (+) low expression, less than 50% of cancer cell express procoagulant to strong intensity; (++) high expression, more than 70% of cancer cells express procoagulant at a strong intensity level. We examined the cancer genome atlas TCGA to check the level of expression of tissue factor in GBM. We selected 451 primary GBMs from the TCGA for which there is IDH1 status is available. Of these 451 GBM tumour samples, 415 are IDH1 wild type and 36 are IDH1-mutant. We extracted level 3 normalized expression data from the TCGA portal and tested the expression levels of F3 between tumours of different IDH1 status using the Kruskal-Wallis rank sum test.
Results
From April 2012 until December 2014, 398 patients with pathologically confirmed GBM were reviewed. Of those, 41 had to be excluded because they were lost to follow up. Therefore, a total of 357 patients were eligible for the current study and those patients were followed for 6 months. Patients were included into the present study after tumour surgery. The mean age of patients in this study was 60.22 years (range 2.4–89.6 years). 239 of 398 (60.05%) patients were male, while females accounted for 159 of 398 (39.94 %).
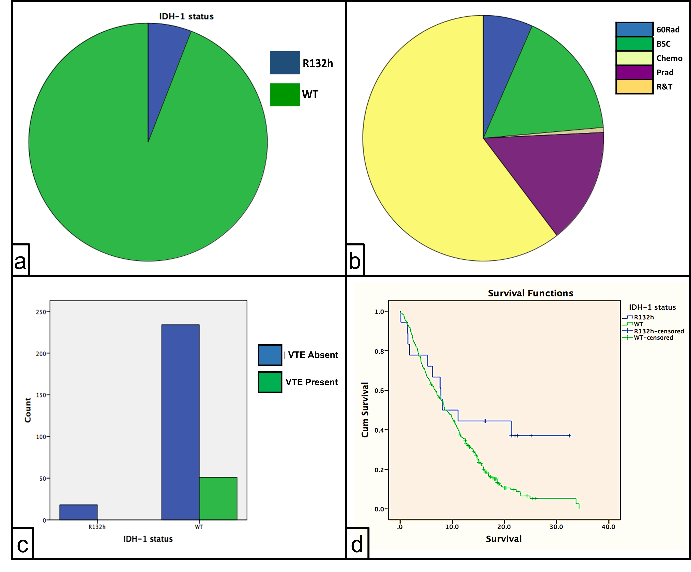
As regards the IDH1 status, 336 (84.4%) were wild type WT, 21 (5.3%) were R132h and 41(10.3%) were missing. 272 (68.3%) patients had craniotomies, 116(29.1%) had biopsies and 10(2.5%) had both. 182 (60.5%) of patients had 60Gy radiotherapy and temozolomide after surgery, 51 (16.9%) had best supportive care, 46 (15.3%) had reduced dose radiotherapy, 20 (6.6%) had 60Gy radiotherapy alone, and 2 (0.7%) had chemotherapy alone (Figure 2).
Figure 2:
a) showing IDH-1 status
b) showing different treatment modalities.
I Thromboembolic events
During follow-up, in total, 51(12.8%) had thromboembolic events; 25 (6.3%) patients had deep venous thrombosis (DVT), 28(7%) developed pulmonary embolism of these 51 patients, 2 patients developed both PE and DVT. Of the 336 WT patients, 51(15.17%) developed thromboembolic events; on the other hand, none of 21 (0%) R132h patients developed any of these events (p-value 0.033 Fisher exact test). As expected, mutant IDH was associated with prolonged patient survival (p=0.024 Log rank). There was no difference in survival or age in patients with / without VTE (p=0.185 Log rank and p=0.733 T-test respectively) (Figure 2).
Figure 3:
a) Representative pancreatic tissue with high TF-expression as a positive control (anti-TF immunostaining) X400.
b) Representative WT IDH HGG case showing that TF expression (brown signal) is abundant and irregular in the tumor specimen and increased in perinecrotic areas and around microvascular proliferates (anti-TF immunostaining) X400.
c) Representative R132H mutant HGG case showing that TF (brown signal) is scanty and focally expressed on cells (anti-TF immunostaining) 400x.
d) Representative R123H mutant LGG with low TF expression on their surface (brown signal) (anti-TF immunostaining)400X.
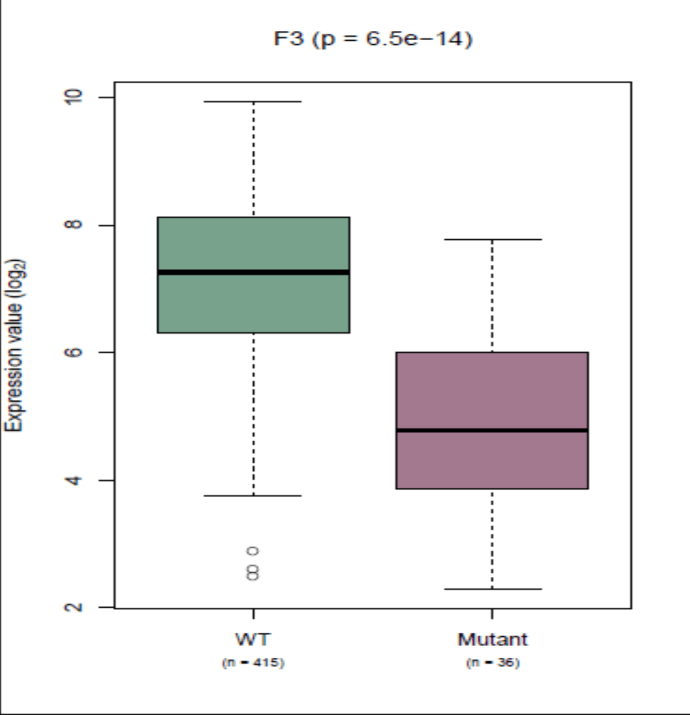
Figure 4: Box plot showing tissue factor expression in both wild type and mutant IDH-1 from TCGA data.
II Immunohistological staining pattern
Factor 3 staining was more intense in WT GBM compared with R132h mutant IDH GBMs or with R132h mutant LGG (Figure 3), averaging ++ compared to +. In tumour specimens staining positive for IDH-1 wild type, expression patterns were much more intense and patchier throughout the tumour. Examining gene expression in the publicly available TCGA cohort dataset, we found a statistically significant increased level of expression of the Factor 3 gene in GBMs with WT IDH compared to GBMs with R132h mutant IDH1. The mean expression in IDH-1 wild type GBM from the TCGA data was 7.14 and in R132h mutant GBM was 4.87 (log2 scale), giving an expression fold change of 2.26 (log2). This was highly statistically significant with a corrected P value of less than 0.0001 (Figure 4).
Discussion
The revolution in genomic studies has enhanced and deepened our understanding of the categorisation of brain tumours [23]. Consequently, studies on GBM in the last few years have shown a paradigm shift from histologic characteristics to molecular and genetic analyses. One of the breakthrough molecular discoveries is the recognition of the key biological role of Isocitrate Dehydrogenase (IDH) mutations.
In our study we found that the majority of GBM patients were wild type (WT) for IDH1, while only (5.3%) were mutant IDH-1 (R132h), echoing previous reports in the literature. Despite the fact that IDH-1 mutation is a key in the process of oncogenesis, it is associated with much better prognosis tumours compared to the wild type IDH-1. Among notable prognostic criteria are co-morbidities associated with disease process in GBM patients. One of these co-morbid conditions is venous thromboembolism (VTE).
Although, thromboembolic events in GBM patients have been the focus of recent research, it remains enigmatic why thromboembolism occurs so frequently in such patients. In this study, VTE occurred in 51 GBM patients (12.8%). Interestingly, none of the mutant type IDH GBM patients were affected while, nearly 15.17 % of the wild type group has suffered such event. These results are slightly lower than those reported by Unruh et al [22]. We searched the Cancer Genome Atlas, and we found that of the coagulation-associated genes, F3 mRNA, encoding for tissue factor has shown a significant rise in WT GBM. In our study we demonstrate higher level of TF expression among patients with wild type IDH, seemingly leading to a higher risk of VTE. This might be explained by IDH mutation induced hypermethylation of genes including the promoter region of the F3 gene [24]. By repressing expression of the F3 promoter through altered DNA methylation, lower local and systemic levels of the pro-coagulant F3 may result, leading to reduced VTE rates. F3 expression in GBM could in general be elevated due to vessel wall damage and hypoxia in rapidly proliferating and necrotic areas of the tumour, thus activating the intrinsic clotting pathway cascade. Similar results were reported and further consolidated with concurrent high activity of TF-MP in peripheral arterial plasma [22]. The study by Unruh et al differed from ours in terms of patient group and the method used to measure expression of TF. The American study recruited different grades of gliomas including low grade while ours are restricted to grade IV Glioma (GBM). We used a murine monoclonal antibody to check the expression of TF while they used a human one. Similar to this study they detected clinically suspected deep venous thrombosis and pulmonary embolism using Doppler and pulmonary C.T angiography and did not undertake systematic screening for sub-clinical VTE.
Given the complex etiological background of VTE, it is thought to be a result of interplay of many interdependent factors rather than completely dependent on one specific factor, so it is likely that many other molecular factors as well as factor 3 and IDH are influential. General condition of the patient, age, co-morbidities, and previous thrombosis are strongly linked to VTE in general [12, 13]. Risk is increased with presence of neurological deficits or immobility in preoperative period [2]. Different haematological markers were studied and used as predictors for development of thromboembolism such as study conducted by Thelar et.al in which he studied 13 different blood biomarkers and found that patients with high platelet count and high P-selectin had the highest risk of developing VTE [25]. Another group, Riedl et al. found that high podoplanin expression induces platelet aggregation, associated with hypercoagulability, and increased risk of VTE [26].
Size and location of tumour were found to significantly alter the risk for VTE e.g. tumours may encroach on motor area and produce paresis which predisposes to thrombosis. Also, steroid use in GBM may increase the risk of thrombosis by causing weakness through steroid induced myopathy. Extent of resection has been found to influence the risk so that patients who underwent biopsy or subtotal resection, are at high risk to develop VTE postoperatively compared with who had total resection [26]. Adjuvant treatments can also increase the risk of thrombosis, with chemotherapy found to increase risk [24]. Anti-angiogenic agents such as Thalidomide was found to be associated with a particularly increased risk [22].
Conclusions
This study illustrates the importance of genetic differences on the clinical behaviour of mutant and wild-type IDH1 GBM in areas other than just overall survival. This may give ways for more effectively identifying GBM patients at high risk of VTE. This could allow for modification of the thromboprophylaxis in patients with GBMs. Potentially less anticoagulant could be offered to patient with mutant type IDH thus reducing the risk of haemorrhagic complications. On the other hand, prolonged use of TED’s stockings and enhanced use of prophylactic low molecular weight heparin in patients with wild type IDH GBMs could be recommended. Further studies are needed to further develop a risk assessment model for VTE in patients with GBM.
Conflicts of Interest
Informed consent was obtained from all individual participants included in the study. There is No Conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 04, Oct 2019Accepted: Tue 05, Nov 2019
Published: Fri 22, Nov 2019
Copyright
© 2023 Ahmed Aly. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2019.04.04
Author Info
Laurence J Glancz Stuart Smith Ahmed Aly Alistair Mccabe Fiona Smith Julie Coventry Kelly Dawson Sadie Boam
Corresponding Author
Ahmed AlyDepartment of Neurosurgery, Nottingham University Hospitals, Nottingham, United Kingdom
Figures & Tables
a) showing IDH-1 status
b) showing different treatment modalities.

a) Representative pancreatic tissue with high TF-expression as a positive control (anti-TF immunostaining) X400.
b) Representative WT IDH HGG case showing that TF expression (brown signal) is abundant and irregular in the tumor specimen and increased in perinecrotic areas and around microvascular proliferates (anti-TF immunostaining) X400.
c) Representative R132H mutant HGG case showing that TF (brown signal) is scanty and focally expressed on cells (anti-TF immunostaining) 400x.
d) Representative R123H mutant LGG with low TF expression on their surface (brown signal) (anti-TF immunostaining)400X.
References
- Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y et al. (2013) CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010 Neuro Oncol 2: 1-56. [Crossref]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10: 459-466. [Crossref]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ et al. (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321: 1807-1812. [Crossref]
- Watanabe T, Vital A, Nobusawa S, Kleihues P, Ohgaki H (2009) Selective acquisition of IDH1 R132C mutations in astrocytomas associated with Li-Fraumeni syndrome. Acta neuropathologica 117: 653-656. [Crossref]
- Pansuriya TC, van Eijk R, d'Adamo P, van Ruler MA, Kuijjer ML et al. (2011) Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet 43: 1256-1261. [Crossref]
- Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S et al. (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483: 474-478. [Crossref]
- 7Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J et al. (2010) Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer cell 18: 553-567. [Crossref]
- Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J et al. (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120: 707-718. [Crossref]
- Brat DJ, Verhaak RG, Aldape KD, Yung WK, Cancer Genome Atlas Research Network et al. (2015) Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med 372: 2481-2498. [Crossref]
- Semrad TJ, O'Donnell R, Wun T, Chew H, Harvey D et al. (2007) Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 106: 601-608. [Crossref]
- Simanek R, Vormittag R, Hassler M, Roessler K, Schwarz M et al. (2007) Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol 9: 89-95. [Crossref]
- Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma. Cancer 89: 640-646. [Crossref]
- Gerber DE, Grossman SA, Streiff MB (2006) Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol 24: 1310-1318. [Crossref]
- Lippi G, Franchini M, Montagnana M, Favaloro EJ. Inherited disorders of blood coagulation (2012) Ann Med 44: 405-418. [Crossref]
- Mann KG (1999) Biochemistry and physiology of blood coagulation. Thromb Haemost 82: 165-174. [Crossref]
- Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P et al. (2007) Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost 97: 119-123. [Crossref]
- Polgar J, Matuskova J, Wagner DD (2005) The P-selectin, tissue factor, coagulation triad. J Thromb Haemost 3:1590-1596. [Crossref]
- Johnson GJ, Leis LA, Bach RR (2009) Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty Thromb Haemost 102: 728-734. [Crossref]
- Lee A, Agnelli G, Büller H, Ginsberg J, Heit J et al. (2001) Dose-response study of recombinant factor VIIa/tissue factor inhibitor recombinant nematode anticoagulant protein c2 in prevention of postoperative venous thromboembolism in patients undergoing total knee replacement. Circulation 104: 74-78. [Crossref]
- Geddings JE, Mackman N (2013) Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 122: 1873-1880. [Crossref]
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC (2013) Epidemiology of cancer-associated venous thrombosis. Blood 122: 1712-1723. [Crossref]
- Unruh D, Schwarze SR, Khoury L, Thomas C, Wu M et al. (2016) Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 132: 917-930. [Crossref]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131: 803-820. [Crossref]
- Turcan S, Rohle D, Goenka A, Walsh LA, Fang F et al. (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483: 479-483. [Crossref]
- Thaler J, Ay C, Kaider A, Reitter EM, Haselböck J et al. (2014) Biomarkers predictive of venous thromboembolism in patients with newly diagnosed high-grade gliomas. Neuro-oncology 16: 1645-1651. [Crossref]
- Riedl J, Preusser M, Nazari PM, Posch F, Panzer S et al. (2017) Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 129: 1831-1839. [Crossref]
