Infranatant Portion of Microfragmented Adipose Tissue: A Promising Source of SVF for the Management of Androgenetic Alopecia
A B S T R A C T
Aim: The purpose of this article is to prove the possibility to transfer a good amount of cells of the SVF (and ADSCs) in the infranatant portion of microfragmented adipose tissue.
Method: The adipose tissue harvesting procedure is performed under local anaesthesia. The adipose tissue was harvested with a 2 mm diameter microperforated cannula with 1 mm side port holes, mounted inside the special patented guide. Both cannula and guide are included in the SEFFIHAIR™ medical device. Once the adipose tissue is harvested, it is gently washed, and it was divided in two specimens: (EMU) the tissue was emulsified with 20 passages from one syringe to another and (CTRL) the tissue didn’t undergo any emulsification.
Results: The emulsification procedure liberated alive and proliferating cells and we observed that the specimens derived with a 1 mm side port hole cannula and then emulsified (EMU) showed a higher number of cells in the infranatant part compared to the one derived from the control tissue without any (1 EMU vs. 1EMU infra).
Conclusion: The use of microcannulas, in combination with a mechanical digestion by an emulsification procedure and centrifuge, could ease SVF cells isolation for regenerative treatment and could also be performed in a medical facility.
Keywords
Androgenetic alopecia, hair loss regenerative therapy, hair transplantation, adipose-derived stem cells, autologous fat transfer, stromal-vascular fraction, regeneration clinical applications
Background
In the last years, several studies proved the efficacy of therapies based on the autologous grafting of adult mesenchymal stem cells to accelerate the healing and regenerative processes of the skin and mesenchymal tissues. In light of these results, some AA proposed the possibility of restoring the hair cycle in male and female androgenetic alopecia (AGA) using autologous fat enriched with SVF [1].
Our study wants to clarify the logic behind the fact that an injection treatment, using only the cells of the SVF, can induce, in AGA prone tissue, such modifications to decelerate or reverse the course of this pathology. A scrupulous analysis has been done on the SEFFIHAIR™ medical device we used to harvest the tissue and on its related procedure. It shows to be effective in obtaining a liquid portion of alive and proliferating ADSCs. These results were obtained by cell characterization which is done with an innovative technology: Celector®. Their consistency proves that this new procedure, simple and safe, can guarantee to this novel therapy an important standardization, necessary for further investigation.
Today regenerative medicine mostly resorts to adipose-derived stem cells (ADSCs) because of their characteristics and easy availability. ADSCs are pluripotent adult progenitor cells derived from embryonic connective tissue. ADSCs carry out their regenerative and immune-modulatory capabilities thanks to the paracrine effects, through trophic factors that show anti-fibrotic, anti-apoptotic and pro-angiogenic activities [2, 3]. ADSCs can also perform their regenerative activity due to their intrinsic capability of transforming into cells of the mesenchymal and endothelial line, promoting tissue reparation [4-6]. Them can also modulate a vast array of immune system cells, among which B and T lymphocytes, neutrophils and “natural killer cells” [7-10].
Based on this, the AA have decided to bring the experience gained in regenerative medicine and skin aging into the trichological field. More specifically we wanted to focus on how their regenerative activity can implement the functions of this tissue leading to the end or the reversal of the follicle miniaturization process.
Androgenetic Alopecia
AGA affects 70% of men and 40% of women at a certain stage of their life. Men typically show a recession of the hairline at the temples and hair loss at the vertex, while women normally show a widespread thinning over the whole upper part of the scalp. Androgenetic hair loss in men begins above the temples and in the center of the scalp. Usually, the strip of hair on the sides and behind the head is maintained.
Androgenetic alopecia in women is colloquially referred to as ‘female pattern baldness’, although its characteristics may also occur in men. It usually causes widespread thinning without a hairline recession and, like the male counterpart, it rarely leads to a complete hair loss. To classify the degrees of alopecia the Hamilton-Norwood Scale is used to measure the degree of baldness in men and the Ludwig Scale to measure the degree of baldness in women.
Pathogenesis and the Role of the Macroenvironment
In genetically predisposed subjects, one of the causes of AGA is dihydrotestosterone (DHT), that derives from testosterone through the action of 5α-reductase. It plays an important role in the miniaturization of the follicle that can lead to the final death of the bulb and an irreversible hair loss. The fundamental pathological process consists in the acceleration, under hormone stimulation, of the mitotic phase of the hair cycle (anagen I-V) and in the consequent reduction of the differentiation phase, which is normally very long. The hair follicle reaches an early transition from the anagen phase, a refractory telogen condition, into a competent telogen phase. The new hairs that derive from an accelerated process will be thinner and shorter.
Many relevant studies revealed that the quiescence of the hair follicle progenitor cells is maintained not only by the immediate niche microenvironment but also by the larger dermal macroenvironment. These studies show that the follicles work in a social network and is not a closed system. An active exchange of cellular signaling, previously implicated in autonomous anagen initiation - bone morphogenic protein (BMP) and WNT - are reused to mediate follicle-to-follicle and macroenvironment-to-follicle communications. It is important to clarify the implications of these studies, in order to emphasize that the macroenvironment includes dermal fibroblasts, skin adipocytes preadipocytes and other extrafollicular components such as intradermal blood vessels, the nerve plexus, and immune cells [11, 12].
Many other pathological processes may take place during AGA evolution and epigenetics may also play a role. However, limiting the observation to the two previous pathological pathways, a recent article argues that DHT could also be a co-mediator of tissue dermal sheath thickening, perifollicular fibrosis, and calcification, three chronic, progressive conditions concomitant with androgenic alopecia progression [13]. Regardless of the reasons behind follicle miniaturization, the histology of AGA prone tissue shows a scalp structure characterized by a restricting follicle growth space, a lack of oxygen and nutrient supplies.
A Clear Target and a Novel Therapy
Based on the above, the AA wishes to emphasize that the uniqueness of the adipose stem cell treatment is to carry out a therapeutic action in the dermal and subcutaneous adipose tissue. Stem cells bring an anti-inflammatory and anti-fibrotic activity in the “follicular macroenvironment” of subjects affected by androgenetic alopecia. Without questioning the validity of the two main therapeutic drugs used to treat AGA (topical minoxidil and oral finasteride), their action mainly occurs in the follicular microenvironment or in the whole organism. Moreover, the efficacy of minoxidil and finasteride can decrease with time as well as the compliance of patients and the rare occurrence of side effects, implemented by a web-mediated nocebo effect, discouraging long term adoption.
The ADSCs therapy acquires, in the light of previous considerations, a precise role in being able to hit an important target until now disregarded. It is also important to outline that an outpatient treatment, such as the adipose stem cells injection, simple and extemporaneous, with nearly no side effects, certainly represents a therapy of great interest. SVF and preadipocytes have demonstrated an important action by introducing the possibility of transforming the dermal macroenvironment. The new cells may induce the activation of follicle stem cells through the secretion of numerous and important Growth Factors, such as platelet-derived growth factor PDGF, vascular endothelial growth factor (VEGF), insulin-like growth factors (binding protein IGFBP-1, and IGFBP-2) [12, 14-16].
Moreover, many studies proved that ADSCs favor the bulb function by stimulating hair follicle stem cells (HFSCs) [17-23]. The association of SVF and platelet-rich plasma (PRP) has proved to be an effective protocol in the treatment of androgenetic alopecia but the standard protocol of autologous regenerative treatment for AGA involves the injection of microfragmented adipose tissue in the scalp [24, 25]. We limited this approach to cases of Secondary Cicatricial Alopecia (SCAs), where the engraftment of mature adipocytes was useful in order to recreate or enhance subdermal thickness [26]. In all other cases of AGA, we believe that the standard protocol does not meet the minimally invasive nature which is an essential requirement for patients seeking regenerative medicine treatments.
In order to successfully introduce ADSCs into treatment plans, we investigated a new protocol (SEFFIHAIR™) that offers the opportunity to implant successfully only the cells of the SVF. We believe that it is essential to use a standard procedure based on a strong preliminary analysis of every single step.
I Facts and Assumptions
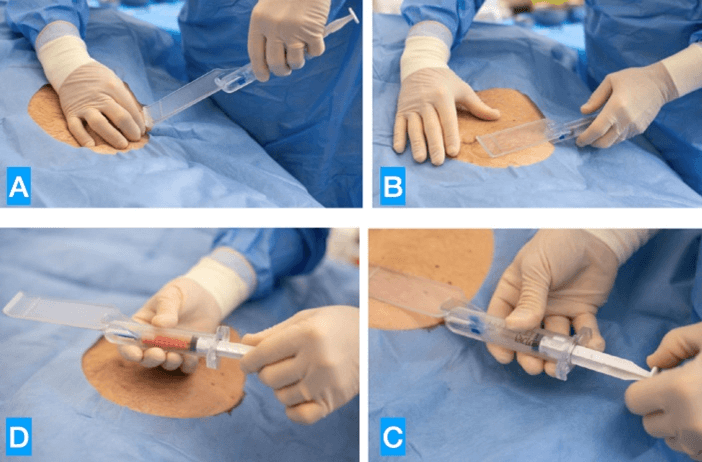
The adipose tissue harvesting procedure is performed under local anaesthesia. The adipose tissue was harvested with a 2 mm diameter microperforated cannula with 1 mm side port holes, mounted inside the special patented guide. Both cannula and guide are included in the SEFFIHAIR™ medical device (produced by SEFFILINE S.r.l. Bologna - Italy) (Figure 1) [27-31].
Figure 1: A) Introduction of the cannula; B) standardized harvesting procedure: 15mm under the skin; C) syringe plunger locked for tissue harvesting; D) harvested tissue.
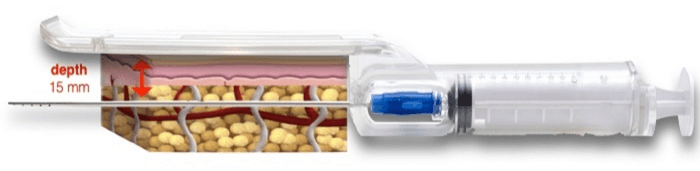
The guide is addressed to standardize the procedure, to guarantee tunneling are performed in the subcutaneous tissue adjacent to the dermis; this layer has been proven to hold more mesenchymal and vascular stem cells (Figure 2) [32, 33].
Figure 2: The guide has standardized tissue harvesting depth of 15 mm.
Once the adipose tissue is harvested, it is gently washed. The tissue harvested and washed was divided in two specimens:
i. EMU: the tissue was emulsified with 20 passages from one syringe to another.
ii. CTRL: the tissue didn’t undergo any emulsification.
Cell Isolation
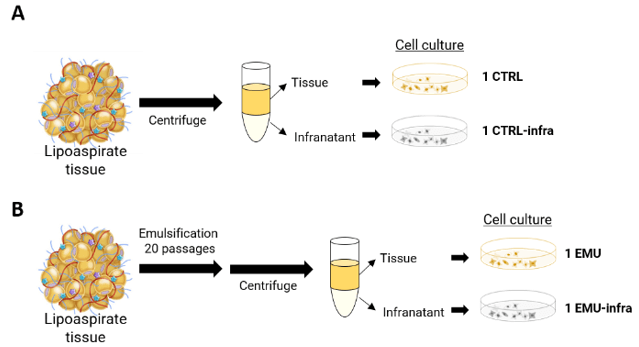
The two specimens (EMU and CTRL) were processed in the lab to quantify the number of adherent and proliferating cells in the tissue and infranatant after centrifugation. The number of infranatant cells derived from the control specimen (CTRL) and emulsified specimen (EMU) were compared to the number of cells recovered from the tissue component in the two specimens. Specimen processing is shown in (Figure 3). Lipoaspirate samples were centrifuged for 10 minutes at 300g. Two different layers were obtained: an upper layer of tissue and a liquid part of infranatant:
i. The infranatant was carefully resuspended and aspirated with a serological pipette, transferred to a new tube, centrifuged for 5 minutes at 300 g to collect cells and then plated in a culture plate.
ii. The adipose tissue layer was resuspended and then divided in 500μL of tissue per 10 cm2 of culture dish.
Each condition was cultured in expansion medium composed of Dulbecco’s Modified Eagle’s Medium High Glucose (DMEM-H), 10% fetal bovine serum (FBS) and 1% Penicillin and Streptomycin (PS) (all material from Lonza, Walkersville, MD, USA).
After 3 days, the medium was replaced with a fresh one; for tissue condition, the medium was replaced, and the tissue was left in the plate to allow full cell migration to plastic surface. Cells were left to grow until they reached 70% of confluence, then were trypsinized and counted. For each sample, cellularity was obtained as the number of counted cells divided per milliliters of infranatant or tissue.
Figure 3: Schematic representation of lipoaspirate processing and culture condition. Lipoaspirate was harvested with Seffihair™ cannula and guide and then equally divided in two. A) One part was treated as control and B) the second one was emulsified. After centrifugation, tissue and infranatant were separated and cellularity (number of cells/ml of tissue and infranatant) was calculated.
Celector® Characterization
Celector® is a patented instrument for live cell-imaging, quality control and label-free separation of cells. The separation happens based only on the cell’s native physical properties: dimensions, morphology and density (Stem Sel Itd., Bologna, Italy) [34]. It could be defined as a “cell chromatographer” that separates cells based on their intrinsic physical characteristics such as dimension, density and membrane conformation [35]. The only force involved is gravity, which doesn’t modify any cell morphology or function. In general, larger cells are eluting at the beginning of the analysis, while smaller cells elute later on. The separation happens without any need of antibodies or additional manipulation. The ability of this new technology is to underline physical and structural differences among cells from the same population.
Fractionation Procedure
At the beginning of each experimental day, decontamination of the fractionation system was performed by flushing the system with cleaning solution. Next, the system was washed copiously with sterile, demineralized water. To block unspecific interaction sites on the plastic walls, the fractionation system was flushed with a sterile coating solution made of 1% bovine serum albumin (BSA) and 1% penicillin/streptomycin (PS) in PBS. Finally, the fractionation system was filled with the sterile mobile phase (all solutions were provided by Stem Sel Ltd, Italy). 00,000 cells in a volume of 100µl were injected and analysed at a flow rate of 1ml/min. Cells from both conditions, adipose tissue and infranatant, were analysed and profiles compared.
Celector® Data Analysis
Celector® profiles were plotted using Excel software. To compare the difference in cell distribution between infranatant-ADSCs and tissue-ADSCs, we calculated the area under the curve in the time interval from the 7th to the 10th minute of analysis using ImageJ software. The value was then expressed in percentage compared to the total area of the fractogram of each condition (%AUC).
Statistical Analysis
Cellularity data were plotted using Graph Pad software and used for statistical analysis.
Results
I Cell Characterization
The emulsification procedure liberated alive and proliferating cells, proving the action of mechanical digestion of the technique, while preserving cell integrity. Cells were recovered from both adipose tissue and infranatant. Cells were able to attach and proliferate to obtain a 70% of confluence in few days of culture. We observed a high heterogeneity in cell recovery, which is probably caused by the inter-biological differences among individuals (age, sex, anatomical location etc.). In general, we observed that the specimens derived with a 1 mm side port hole cannula and then emulsified (EMU) showed a higher number of cells in the infranatant part compared to the one derived from the control tissue without any (1 EMU vs. 1EMU infra).
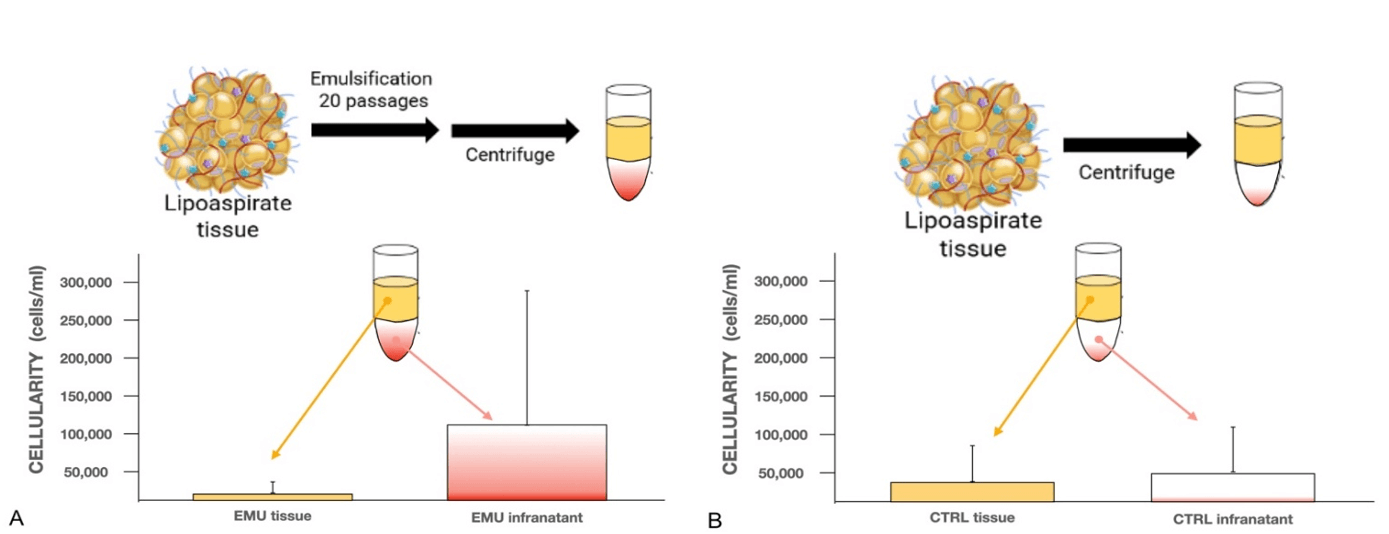
In the control specimens (CTRL) the cellularity in the tissue and in the infranatant was similar (1 CTRL vs 1 CTRL infra) (Figure 4).
Figure 4: A) Emulsified tissue and Infranatant cellularity (EMU tissue – EMU infranatant B) tissue and Infranatant cellularity without emulsification (CTRL tissue – CTRL infranatant). Cellularity is expressed by the number of counted cells after first trypsinization divided by the number of milliliters of tissue or infranatant.
The higher cellularity of the emulsified infranatant (EMU infra) could be explained by the additional digestion based on the mechanical action of the emulsification step, that liberates many more cells in the environment (Figure 5).
Figure 5: EMU specimens: the emulsification acts as a mechanical digestion.
The use of SEFFIHAIR™ cannula already performs a mechanical tissue digestion, thanks to the action of the small size side port holes. During aspiration there is a simultaneous mincing of the tissue: some progenitor cells are present in the aqueous part and some are still entrapped in the tissue. However, the mincing procedure of the cannula allows loosening of the extracellular matrix of tissue with consequent cells release in the following days.
II Celector® Profile
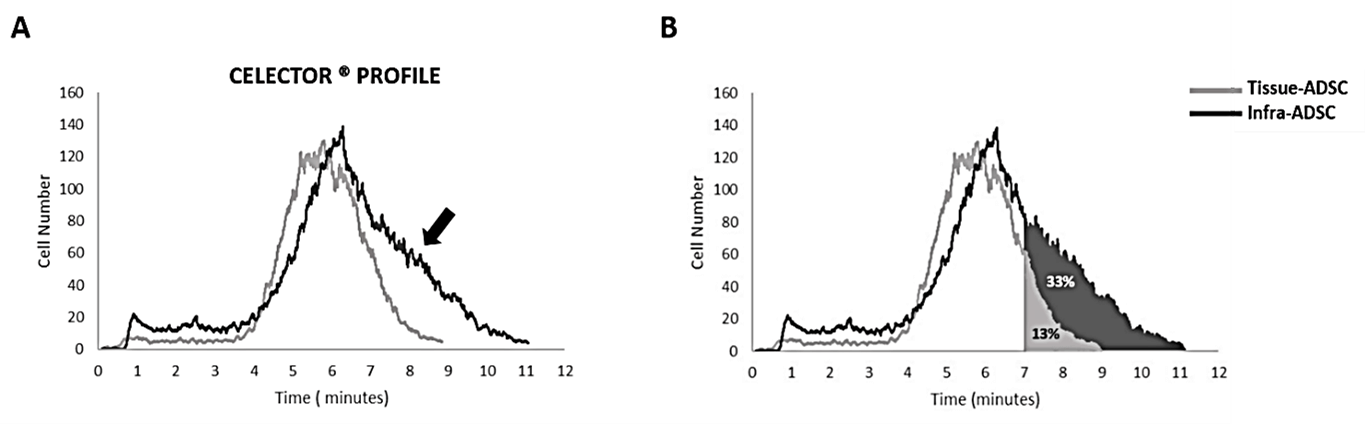
Single-cell solution from expanded culture is injected into Celector® and a stream of biocompatible fluid carries them in the capillary channel. Here, cells acquire specific eluting velocity depending on their physical characteristics: larger and denser cells elute earlier than smaller cells. A microcamera detector placed at the exit of the capillary channel (USB 2.0 board-level camera-mvBlueFox-MLC, Matrix Vision, Oppenweiler, Germany) monitors the elution process, generating a recorded plot of the eluted cell number as a function of time (fractogram). The evolution of the curve from tissue-ADSCs showed a homogeneous population, with a normal cell distribution. Instead, when infranatant-ADSCs were analysed, the profile showed a larger cell distribution, having a broader base of the fractogram, with a majority of cells eluting in the last part of the analysis (tissue-ADSC: 4-8 minutes; infranatant-ADSCs: 4-10 minutes). To quantify this evidence, we calculated the percentage of the area under the curve (%AUC) for both profiles in the last eluate (from 7 to 10 minutes).
The % AUC of infranatant-ADSCs was 33% and for tissue-ADSC was 13%. Therefore, there is an increase of 20% of smaller cells in the infranatant-ADSCs populations compared to tissue-ADSCs population. This difference could be explained by the principle that cells derived from the cultured tissue belong to the same cell type, whereas cells liberated in the infranatant by the mechanical action of liposuction using SEFFIHAIR™ cannula are a mixed population, as also underlined by the presence of a second peak in the curve (Figure 6).
Figure 6: Celector® Profile. A) Representative profiles from tissue-ADSCs and infranatant-ADSCs. Tissue-ADSCs eluted from minute 4 until minute 8, while infranatant-ADSCs eluted from minute 4 until minute 10. The heterogeneity of the infranatant-ADSCs is shown by the presence of a second peak (indicated by the arrow). B) Schematic representation of the area under the curve (AUC) for both profiles showing their relative percentage.
Conclusion
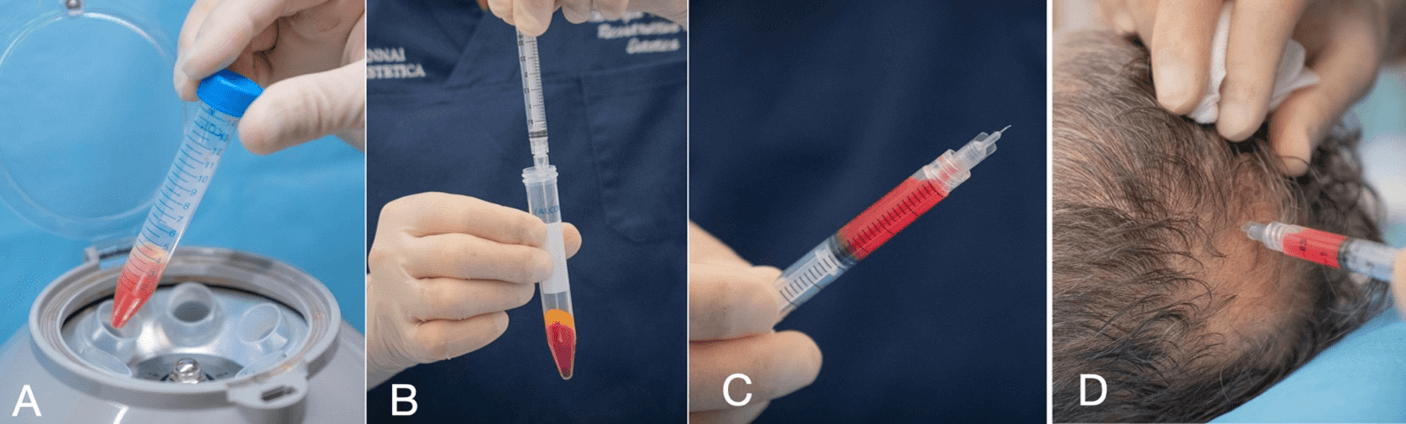
Enzymatic digestion of the adipose tissue is a gold standard procedure for progenitor cells/stem cells isolation. This procedure is time consuming; it could have an impact on cell viability and yield, and it is mandatory to perform it in equipped laboratories in order to carry out all procedures in a sterile environment. The use of microcannulas, in combination with a mechanical digestion by an emulsification procedure and centrifuge, could ease SVF cells isolation for regenerative treatment and could also be performed in a medical facility (Figure 7).
Figure 7: Ease SVF cells: A) guided tissue harvesting; B) emulsification; C) centrifuge 10 min 300g.
We proved that both the emulsified tissue and the infranatant, derived from the emulsification procedure, contained viable cells (Figure 8).
Figure 8: In the EMU specimens, after centrifuging for 10 min 300g, the SVF cells move from tissue to infranatant.
When cellularity was compared, infranatant from emulsification step (EMU) contained a higher number of cells, proving the digestion activity of the emulsification step and its utility in different regenerative treatments. Cells in the infranatant were already shown by Yoshimura et al., but they derived stem cells by Ficoll separation, adding therefore another manipulation step to the entire procedure [36].
We wanted to see if the emulsification led to a release of different cells compared to adipose tissue itself. Based on the Celector® profile, we concluded that infranatant cells were more heterogeneous compared to tissue-ADSCs. Heterogeneity is correlated to the base of the fractogram, the time of analysis. The wider the base of the fractogram, the higher the heterogeneity of the sample, because different types of cells acquire different velocity inside the fractionation channel and therefore elute at different time. infranatant-ADSCs showed smaller cells compared to tissue-derived ADSC. This result proves the ability of a higher cell release of the liposuction procedure in combination with the emulsification step which allowed indeed a better digestion of the tissue. Further studies are needed to deepen our understanding of the composition of this interesting cell population for regenerative purposes.
This study proved that a good amount of SVF cells are present and viable in the infranatant component of the emulsified tissue (infranatant – EMU). According to many AA, SVF and ADSCs could play a fundamental role in the AGA treatment. Moreover, this study opens the possibility to use only the infranatant component of the emulsified microfragmented adipose tissue without any need of enzymatic digestion (Figure 9). In the future we would like to outline further the role of ADSCs in the context of existing therapies to prevent the idea that a magic wand is coming onto the market, like it disgracefully happened with previous injectable therapies (PRP). Our method is not only an important step for a proper evaluation of the clinical results, but it also offers a viable option for an easy and repeatable therapy in AGA treatment.
Figure 9: A) Centrifuge 10 min 300rpm; B) harvesting infranatant; C) infranatant ready to be injected; D) injection in the scalp with a 27G 4mm long needle.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 14, Jan 2021Accepted: Thu 28, Jan 2021
Published: Fri 12, Feb 2021
Copyright
© 2023 Alessandro Gennai. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2021.01.01
Author Info
Alessandro Gennai Piero Tesauro Mattia Colli Silvia Zia Barbara Roda Andrea Zattoni
Corresponding Author
Alessandro GennaiPlastic Surgeon, Private Practice, Bologna, Italy
Figures & Tables
References
- Perez Meza D, Ziering C, Sforza M, Krishnan G, Ball E et al. (2017) Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning 10: 1-10. [Crossref]
- Singer NG, Caplan AI (2011) Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6: 457-478. [Crossref]
- Delarosa O, Dalemans W, Lombardo E (2012) Mesenchymal stem cells as therapeutic agents of inflammatory and autoimmune diseases. Curr Opin Biotechnol 23: 978-983. [Crossref]
- Rossi AF, Ricci F, Vignoli F, Marchionni C, Valente S et al. (2016) In vitro multilineage potential and immunomodulatory properties of adipose derived stromal/stem cells obtained from nanofat lipoaspirates. CellR4 4: e2212.
- Planat Benard V, Silvestre JS, Cousin B, André M, Nibbelink M et al. (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109: 656-663. [Crossref]
- Rossi M, Roda B, Zia S, Vigliotta I, Zannini C et al. (2018) Characterisation of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesthet Surg J 40: 679-690. [Crossref]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R et al. (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigenspecific T cells to their cognate peptide. Blood 101: 3722-3729. [Crossref]
- Delarosa O, Sánchez Correa B, Morgado S, Ramírez C, del Río B et al. (2012) Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev 21: 1333-1343. [Crossref]
- Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB et al. (2014) Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20: 633-641. [Crossref]
- Puissant C, Barreau P, Bourin P, Clavel C, Corre J et al. (2005) Immunomodulatory e ect of human adipose tissue-derived adult stem cells: compar- ison with bone marrow mesenchymal stem cells. Br J Haematol 129: 118-129. [Crossref]
- Plikus MV (2012) New Activators and Inhibitors in the Hair Cycle Clock: Targeting Stem Cells’ State of Competence. J Invest Dermatol 132: 1321-1324. [Crossref]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M et al. (2011) Adipocyte Lineage Cells Contribute to the Skin Stem Cell Niche to Drive Hair Cycling Cell 146:761-771. [Crossref]
- English RS (2018) A hypothetical pathogenesis model for androgenic alopecia: clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med Hypotheses 111: 73-81. [Crossref]
- Won CH, Yoo HG, Kwon OS, Sung MY, Kang YJ et al. (2010) Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci 57: 134-137. [Crossref]
- Anderi R, Makdissy N, Azar A, Rizk F, Hamade A (2018) Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther 9: 141. [Crossref]
- Park BS, Kim WS, Choi JS, Kim HK, Won JH et al. (2010) Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res 31: 27-34. [Crossref]
- Won CH, Park GH, Wu X, Tran TN, Park KY et al. (2010) The Basic Mechanism of Hair Growth Stimulation by Adipose-derived Stem Cells and Their Secretory Factors. Curr Stem Cell Res Ther 12: 535-543. [Crossref]
- Shin H, Won CH, Chung WK, Park BS (2017) Up-to-date Clinical Trials of Hair Regeneration Using Conditioned Media of Adipose-Derived Stem Cells in Male and Female Pattern Hair Loss. Curr Stem Cell Res Ther 12: 524-530. [Crossref]
- Shin H, Ryu HH, Kwon O, Park BS, Jo SJ (2015) Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol 54: 730-735. [Crossref]
- Owczarczyk Saczonek A, Wociór A, Placek W, Maksymowicz W (2017) Wojtkiewicz The Use of Adipose-Derived Stem Cells in Selected Skin Diseases (Vitiligo, Alopecia, and Nonhealing Wounds). J Stem Cells Int 2017: 474070. [Crossref]
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, Abdou SM, El Attar YA et al. (2018) Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat 29: 431-440. [Crossref]
- Stem Cell Center. Molecular Cell Biology, Genetics, and Development Program Yale University, 219 Prospect St., New Haven, CT 06520, USA.
- Epstein GK, Epstein JS (2018) Mesenchymal Stem Cells and Stromal Vascular Fraction for Hair Loss: Current Status. Facial Plast Surg Clin North Am 26: 503-511. [Crossref]
- Stevens HP, Donners S, de Bruijn J (2018) Introducing Platelet-Rich Stroma: Platelet-Rich Plasma (PRP) and Stromal Vascular Fraction (SVF) Combined for the Treatment of Androgenetic Alopecia. Aesthet Surg J 38: 811-822. [Crossref]
- Butt G, Hussain I, Ahmad FJ, Choudhery MS (2019) Stromal vascular fraction-enriched platelet-rich plasma therapy reverses the effects of androgenetic alopecia. J Cosmet Dermatol. [Crossref]
- Tesauro P, Trivisonno A, Gennai A, Marliani A, Clauser L (2020) Hair Transplantation in Cicatricial Alopecia: The Role of Autologous Fat Transfer. Int J Regener Med 3: 11.
- Gennai A, Zia S, Roda B, Maggio A, Bonsi Alviano et al. (2020) SEFFI (Superficial Enhanced Fluid Fat Injection) for Aesthetic and Clinical Regenerative Treatments. Global J Dermatol Venereol 8: 32-40.
- Gennai A, Bernardini FP (2017) Superficial enhanced fluid fat injection (SEFFI and MicroSEFFI) in facial rejuvenation. CellR4 5: e223.
- Gennai A, Zambelli A, Repaci E, Quarto R, Baldelli I et al. (2016) Skin Rejuvenation and Volume Enhancement with the Micro Superficial Enhanced Fluid Fat Injection (M-SEFFI) for Skin Aging of Periocular and Perioral Regions. Aesth Surg J 37: 14-23. [Crossref]
- Bernardini FP, Gennai A (2015) Superficial Enhanced Fluid Fat Injection for Volume Restoration and Skin Regeneration of the Periocular Aesthetic Unit. An Improved Fat Grafting Technique to enhance the beauty of the eye. JAMA Plastic Facial Surg 18.
- Bernardini FP, Gennai A, Izzo L, Zambelli A, Repaci E et al. (2015) Superficial Enhanced Fluid Fat Injection (SEFFI) to Correct Volume Defects and Skin Aging of the Face and Periocular Region. Aesthet Surg J 35: 504-515. [Crossref]
- Clauser L, Lucchi A, Trussardi TI, Gardin C, Zavan B (2018) Autologous fat transfer for facial augmentation and regeneration: Role of mesenchymal stem cells. Atlas Oral Maxillofac Surg Clin North Am 26: 25-32. [Crossref]
- Trivisonno A, Di Rocco G, Cannistra C, Finocchi V, Farr ST et al. (2014) Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet Surg J 34: 601-613. [Crossref]
- Rossi M, Roda B, Zia S, Vigliotta I, Zannini C et al. (2018) Characterization of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesth Surg J 40: 679-690. [Crossref]
- Roda B, Lanzoni G, Alviano F, Zattoni A, Costa R et al. () A novel stem cell tag-less sorting method. Stem Cell Rev Rep 5: 420-427. [Crossref]
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y et al. (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208: 64-76. [Crossref]
