Journals
Infected Abdominal Cystic Lymphangioma: A Rare Cause of Inflammatory Acute Abdom
A B S T R A C T
Background: Cystic lymphangioma (CL) results from congenital malformation of the lymphatic channels found especially in children, being rarely diagnosed in adults. Intra-abdominal location is unusual, and only exceptionally may the abdominal cavity be infected.
Case report: We report the case of a 28-year-old man with retroperitoneal CL who presented with acute abdominal pain, fever, signs of sudden decompression, and leukocytosis. Imaging studies showed an intra-abdominal collection of fat density within the cyst. Presence of fat within an intra-abdominal cystic mass is suggestive of a dermoid cyst (mature cystic teratoma), CL, or lymphocele. At laparotomy, the tumor was found in the retroperitoneum and completely resected. Histopathology of the cystic lesion identified a fibrinopurulent exudate and lymphatic channels interspersed with lymphoid stroma, while immunohistochemistry showed positive staining for D2-40 and CD34, confirming the diagnosis of infected retroperitoneal cyst.
Conclusions: Presentation as an acute abdomen is uncommon and surgery is the treatment of choice for symptomatic patients with CL. The prognosis is excellent, and recurrence rates are extremely low if the cyst is completely excised.
Keywords
cystic lymphangioma, infected retroperitoneal cyst, acute abdomen.
Introduction
Cystic lymphangioma (CL) is a rare benign disease resulting from congenital malformation of the lymphatic channels. CL is more commonly found in children, being rarely diagnosed in adults [1]. Typical locations are the head, neck, and axilla, while intra-abdominal locations (mesentery, omentum, and retroperitoneum) are extremely unusual. The patient’s average age at presentation is 2 years; approximately 60% are diagnosed before the fifth year of life [2]. Clinical manifestations are polymorphic, and the most common are abdominal mass and abdominal distension [3, 4]. Diagnostic suspicion is based on imaging findings, but the diagnosis is confirmed by histopathology.
Case Reports
A 28-year-old previously healthy patient referred acute-onset epigastric pain radiating to the left iliac fossa about 6 hours after onset, progressing to abdominal distension and fever. He denied other digestive symptoms. Physical examination revealed pain on palpation of the left iliac fossa with sudden painful decompression. Laboratory tests showed a leukocyte count of 16,700 cells without any bands, myelocytes, or metamyelocytes.
Abdominal computed tomography (CT) scan showed an encapsulated collection of fat density and fluid content measuring 4.8 x 4.8 cm, with adjacent mesenteric fat infiltration and thickening of left anterior pararenal and lateroconal fasciae, suggesting a dermoid or mesenteric cyst.
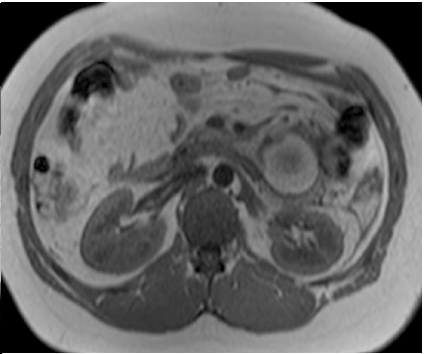
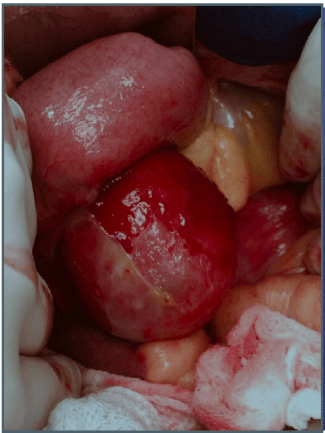
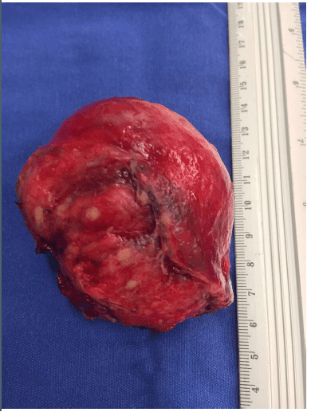
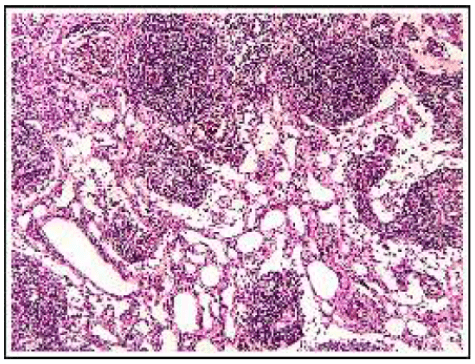
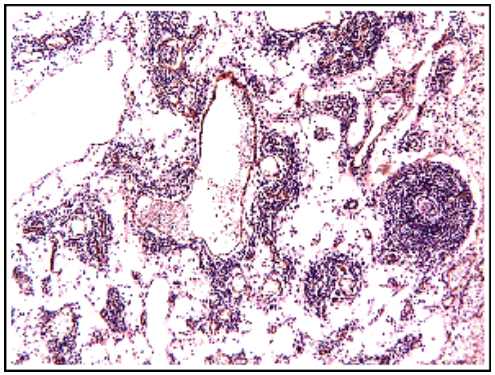
Abdominal magnetic resonance imaging showed a cystic lesion located superficial to the left anterior renal fascia and lateral to the duodenojejunal junction, measuring approximately 5.0 cm in diameter. The lesion exhibited walls with decreased signal intensity on both T1- and T2-weighted images (Figure 1) and content with increased signal intensity on both T1- and T2-weighted images, with a marked decrease in signal intensity on out-of-phase T1-weighted images with fat saturation, compatible with fat content accompanied by a small amount of fluid. No water diffusion restriction was observed. After intravenous injection of the paramagnetic contrast agent gadolinium, moderate enhancement of the lesion walls was noted. Intravenous antibiotic therapy was started, and exploratory laparotomy was indicated. It revealed a cystic tumor in the retroperitoneum, adjacent to the third portion of the duodenum, measuring 9.0 cm. The tumor was isolated from other organs and completely excised (Figure 2). Gross examination of the specimen revealed a violaceous nodule with fibrin adhered to its external surface, weighing 70 g and measuring 7.0 x 6.0 x 5.0 cm. Sections showed a cystic cavity filled with thick material and with a smooth surface, measuring 6.0 x 5.0 x 4.5 cm, at 1.0 cm from the resection margin. The histopathological diagnosis was cystic abscess lesion with abundant fibrinopurulent exudate, without epithelial lining, closely related at the periphery to vascular malformation, consisting of lymphatic channels interspersed with lymphoid stroma, supporting the hypothesis of CL with suppurative inflammation (infection). Immunohistochemistry showed positive staining for D2-40 antibody in the permeated lymphatic vessels and positive staining for CD34 in the vascular spaces. The immunohistochemical profile was compatible with angiolymphatic malformation (Figure 3).
Figure 1A: Axial T2-weighted images (HASTE and TSE), with and without fat saturation, showing a lesion with increased signal intensity, suggesting fluid and/or fat content, accompanied by edema of adjacent fat planes.
Figure 1B: Axial in-phase and out-of-phase T1-weighted images (GRE). Note the marked decrease in signal intensity on the out-of-phase image, characterizing a predominance of fat content.
Figure 2A: Cystic lesion adjacent to the third portion of the duodenum.
Figure 2B: Complete resection of the tumor, measuring 9.0 cm.
Figure 3A: Histopathology showing a cystic tumor with abundant fibrinosuppurative exudate, containing lymphatic channels interspersed with lymphoid stroma.
Figure 3B: Immunohistochemistry showing immunoreactivity for CD34, suggesting angiolymphatic malformation.
CLs are rare hamartomas whose etiology remains unclear. The main theory is that CLs arise from sequestration of lymphatic tissue during embryonic development, with disrupted communication between the lymphatic vessels and venous system [1]. Half of the lesions are present at birth. About 90% will grow until 2 years of age, being rare in adults [2]. Our patient was 28 years old. The most common sites are the head, neck, and axillary region, being rarely found in the abdominal cavity [3].
CLs account for less than 10% of abdominal cystic lesions in adults, being preferably located in the mesentery, while the retroperitoneum is a rare location [1]. The CL of the case reported here was located in the retroperitoneum, close to the third portion of the duodenum. Most patients have no complaints, becoming symptomatic only when the cyst increases in size. The main clinical manifestation is a palpable mass. Hebra et al. showed, in a series of 22 cases, that half of the lesions presented as asymptomatic abdominal masses, while the remaining 11 cases presented as chronic abdominal pain [5]. In the study by Makni et al., 75% of patients (15 cases) had abdominal pain, while a palpable mass was noted in 60% of patients (12 cases) [6]. The mean cyst size was 10 cm. However, our patient had an unusual clinical manifestation of CL, with intense acute abdominal pain, fever, signs of sudden painful decompression, and leukocytosis - signs and symptoms compatible with inflammatory acute abdomen. Presentation as an acute abdomen is uncommon, according to data from the literature [2, 7].
The diagnostic imaging method of choice is abdominal ultrasound, but in acute cases with infection or hemorrhage, CT provides more accurate information [8]. Fat can be easily defined on CT if present in an amount sufficient to decrease density in relation to the fluid content, as observed in our case. The differential diagnosis of a cystic mass containing fat includes dermoid cyst, CL, and lymphocele. Dermoid cysts are characterized by a cystic mass containing cholesterol. Nearly all cases of mature cystic teratomas of the ovary contain sebaceous material. It may be difficult to differentiate between a dermoid cyst and CL in the presence of hemorrhage or infection [9]. Other differential diagnoses include cystic mesothelioma, leiomyoma or leiomyosarcoma, liposarcoma, enteric cysts, and digestive duplication [6, 10].
Additional complications may occur, such as intestinal obstruction, volvulus, spontaneous rupture of the cyst, and gastrointestinal bleeding. Calcifications are rarely seen in CL. Surgery is the treatment of choice for symptomatic abdominal CL, while watchful waiting is indicated in patients without complaints. Simple aspiration is not indicated due to the high recurrence rate. Recurrence occurs in 40-50% of cases of incomplete resection, achieving much lower rates when surgical treatment is considered macroscopically complete [6]. The prognosis is excellent and directly related to the complete resection of CL [6, 7].
In conclusion, CL is a benign tumor of the lymphatic system that should be treated conservatively in asymptomatic patients, while surgical treatment is indicated for symptomatic patients due to increased tumor mass or presence of complications. Complete resection of the cyst is the recommended approach, which is directly related to low recurrence and excellent prognosis.
Article Info
Article Type
Case ReportPublication history
Received: Tue 22, Jan 2019Accepted: Fri 15, Feb 2019
Published: Mon 11, Mar 2019
Copyright
© 2023 Carlos Eduardo Oliveira dos Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.01.008
Author Info
Angélica Maria Lucchese Antonio Nocchi Kalil Fernanda Onofrio Rodrigo Zanatta Ribeiro
Corresponding Author
Carlos Eduardo Oliveira dos SantosDepartment of Gastroenterology and Endoscopy, Santa Casa de Caridade Hospital, Bagé, RS, Brazil
Figures & Tables

References
- Rifki JS, Adraoui J, Khaiz D, Chehad F, Lakhloufi A (2004) Le lymphangiome kystique rétropéritonéal. ProgUrol 14: 548-550.
- Konen O, Rathaus V, Dlugy E, Freud E, Kessler A, et al. (2002) Childhood abdominal cystic lymphangioma. Pediatr Radiol 32: 88-94. [Crossref]
- Losanoff JE, Richman BW, El-Sherif A, Rider KD, Jones JW (2003) Mesenteric cystic lymphangioma. J Am Coll Surg 196: 598-603.
- Rekhi BM, Esselstyn CB Jr, Levy I, Mercer RD (1972) Retroperitoneal cystic lymphangioma. Report of two cases and review of the literature. Cleve Clin Q 39: 125-128. [Crossref]
- Hebra A, Brown MF, McGeehin KM, Ross AJ 3rd (1993) Mesenteric, omental, and retroperitoneal cysts in children: a clinical study of 22 cases. South Med J 86: 173-176. [Crossref]
- Makni A, Chebbi F, Fetirich F, Ksantini R, Bedioui H, et al. (2012) Surgical Management of Intra-Abdominal Cystic Lymphangioma. Report of 20 Cases. World J Surg 36: 1037-1043. [Crossref]
- Talukdar S, Alagaratnam S, Sinha A, Thorn CC, Elton C (2011) Giant cystic lymphangioma in childhood: a rare differential for the acute abdomen. BMJ Case Rep 2011. [Crossref]
- Davidson AJ, Hartman DS (1990) Lymphangioma of the retroperitoneum: CT and sonographic characteristics. Radiology 175: 507-510. [Crossref]
- Yoo E, Kim MJ, Kim KW, Chung JJ, Kim SH, et al. (2006) A case of mesenteric cystic lymphangioma: fat saturation and chemical shift MR imaging. J Magn Reson Imaging 23: 77-80. [Crossref]
- Iyer R, Eftekhari F, Varma D, Jaffe N (1993) Cystic retroperitoneal lymphangioma: CT, ultrasound and MR findings. Pediatr Radiol 23: 305-306. [Crossref]
