Individual Dose Response and Radiation Origin of Childhood and Adolescent Thyroid Cancer in Fukushima, Japan
A B S T R A C T
Background: After the Fukushima Nuclear Power Plant accident in 2011, thyroid ultrasound screening was carried out as part of the Fukushima health management survey (FHMS) on all residents of ages≤18 years at the accident. The results of the 1st (2011-2013) and 2nd (2014-2015) round screening showed dozens of times excess thyroid cancer detection compared to the expected from the cancer registry. Both FHMS and the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) summarized that thyroid cancer cases detected in Fukushima were unlikely to be attributed to radiation exposure from the absence of dose-response relation. We elucidated the response of thyroid cancer incidence to individual external doses and found the dominant radiation effect on the increased thyroid cancer incidence after the accident.
Method: Association between average individual external dose based on FHMS basic survey and thyroid cancer incidence rate of dose groups was analysed by the linear regression analysis. Thyroid dose estimated in UNSCEAR 2020/2021 for 10-year-old children was used for comparison.
Results: Incidence proportions of dose groups in the 2nd round showed a linear response to mean individual external dose under 2.5 mSv range. A rough estimate of the excess relative risk per gray (Gy) was ERR/Gy =213 (95%CI: 129, 297) (p=0.02).
Conclusion: Childhood and adolescent thyroid cancer in Fukushima was associated with individual external dose estimated in FHMS basic survey. Increased childhood and adolescent thyroid cancer in Fukushima could most probably be attributed to radiation exposure from the nuclear accident.
Keywords
Childhood thyroid cancer, individual dose-response, radiation exposure, excess relative risk, radiation origin, age distribution, Fukushima nuclear accident
Introduction
After the radioactive fallout from Fukushima Daiichi Nuclear Power Plant on March 11, 2011, Fukushima Prefecture initiated thyroid ultrasound screening for all residents aged ≤18 years at the time of the accident. The Fukushima Health Management Survey (FHMS) conducted by Fukushima Medical University (FMU) reported that 266 confirmed or suspected cancer cases were detected from the 1st to 5th round screening (fiscal year FY2011-2021) and a milestone screening every five years after 20 (confirmed on 25.10.2021) [1, 2]. Cancer cases were nearly 300 if confirmed thyroid cancer cases outside FHMS were included.
Although the FHMS committee evaluated that detected thyroid cancer cases in the 1st and 2nd rounds were dozens of times higher than those estimated from the Japanese cancer statistics, the conclusion was that no association could be found between radiation exposure and thyroid cancer detected in the 1st and 2nd round thyroid screening [2, 3]. A decade after the Fukushima Nuclear Power Plant accident, the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) updated dose estimates to the public, which were decreased from the previous report (UNSCEAR 2013) and concluded that future adverse health effects directly related to radiation exposure were unlikely to be discernible on 9 March 2021 [4, 5].
Three research groups showed associations between area dose and thyroid cancer incidence in the 2nd round and the 1st +2nd round [6-9]. Kato found high associations between ares dose and childhood thyroid cancer in the 2nd and 1st+2nd rounds [6, 7]. Yamamoto et al. reported that the thyroid cancer detection rate per exposed person-years and the radiation dose rate in 59 municipalities in Fukushima prefecture showed statistically significant dose-response relationships for the 1st, 2nd, and 1st+2nd examination participants [8]. They concluded that radiation contamination due to the accident was positively associated with thyroid cancer detection rates. Ohira et al. of the FMU group found no significant exposure effects [10, 11].
In this paper, the dependence of thyroid cancer incidence proportion of dose groups in the 2nd round on the mean individual external dose was studied. The possible radiation origin of childhood thyroid cancer after the nuclear accident in Fukushima was examined from the cancer registry in Japan and the age distribution of thyroid cancer cases as compared with the Chernobyl cases.
Method
I Thyroid Ultrasound Screening and Study Subjects
Thyroid ultrasound screening was performed as part of the FHMS for all 367,649 residents aged ≤18 years at the accident, and 115 and 71 confirmed or suspected cancer cases were detected among 300,473 and 270,511 examinees in the 1st (FY2011-2013) and 2nd (FY2014-2015) round screening, respectively [1, 2]. Among 151 positive cases in fine-needle aspiration cytology (FNAC), 150 cases (99.3%) were confirmed to be malignant by surgery. In this paper, positive cases in FNAC were defined as cancer cases. Individual dose dependence of thyroid cancer was studied here based on the radiation dose data of 36 cancer cases in the FHMS report and the distribution of individual external dose of 108,980 examinees with external dose data estimated for the first four months after the accident [1, 10]. The thyroid examination dataset used in this paper was deidentified and publicly available, so no ethical review was required.
II Dose Estimation
Individual external dose based on personal behaviours was estimated in the basic survey of FHMS [1]. Individual dose dependence of thyroid cancer incidence was studied by dividing 108,980 examinees into three external dose groups, <1mSv, 1–2mSv, and ≥2mSv [10]. The correlation between the average external dose by the municipality based on the FHMS basic survey report and thyroid dose of 10-year-old children of the municipality estimated in the UNSCEAR 2020/2021 report was studied, where the external dose for each municipality was an average external dose weighted by the number of the basic survey respondents [1, 4, 7, 12].
III Statistical Analysis
Dose-response of thyroid cancer incidence proportion (IP) was analysed by the linear risk model of the form IP= r0 + r1* dose, and the relative risk RR, the ratio of IP compared to IP at zero-dose was analysed by the linear excess relative risk (ERR) model of the form RR= IP / r0 =1 + (r1/r0) * dose, where the slope coefficient r1/r0 gave ERR per unit dose (Gy or mSv) [13, 14]. Dose-response of IP and RR was analysed by the linear regression analysis of Microsoft Excel 2019 MSO (2112).
Results
I Association Between Individual Dose and Risk of Thyroid Cancer
Individual dose dependence of thyroid cancer was studied from the radiation dose data of 36 cancer cases: 15 (dose < 1 mSv), 16 (1–2 mSv), and 5 (≥2 mSv) in the 2nd round of FHMS report, and the individual external dose distribution of the 108,980 participants estimated by Ohira et al. [1, 10]. They reported that the average external dose of 1.1 mSv for thyroid cancer cases was higher than the average dose of 0.92 mSv for no cancer cases, and the percentages of examinees with dose ≥1 mSv in groups with and without thyroid cancer were 58% and 42%, respectively (Table 1 in [10]). They also reported that the incidence odds ratio of participants with individual dose ≥ 2 mSv compared to dose group <2 mSv was 2.09 (95%CI: 0·81-5·40). All the data suggested a positive dose-response of thyroid cancer incidence.
Table
1:
Individual external dose group, mean external dose, converted thyroid dose,
thyroid cancer and non-thyroid cancer cases. Incidence, incidence odds ratio,
and relative risk compared to the extrapolated risk at zero-dose in the
second-round screening.
|
Individual external
Dose Group |
Mean external dose mSv |
Converted thyroid
dose Gy |
Thyroid cancer |
Non thyroid
cancer |
Total examinee |
Incidence / 104 |
Incidence Odds
ratio (95%CI) |
Relative risk
(95%CI) |
|
≥ 2 mSv |
2.57 |
0.0197 |
5 |
7803 |
7808 |
6.40 |
2.7 (1.0, 7.4) |
4.7 (1.7, 12.9) |
|
1~2mSv |
1.5 |
0.0115 |
16 |
37953 |
37969 |
4.21 |
1.8 (0.9, 3.6) |
3.1 (1.5, 6.3) |
|
< 1 mSv |
0.5 |
0.0038 |
15 |
63188 |
63203 |
2.37 |
1(Ref.) |
1.7 (0.9, 3.6) |
|
0 mSv |
0 |
0 |
|
|
|
|
|
1 (Ref.) |
|
Sum/Average |
|
|
36 |
108944 |
108980 |
3.30 |
|
The numbers of participants in dose groups, the incidence of thyroid cancer, and incidence odds ratios were calculated by using the above data (Table 1). The mean doses of the first two groups (<1 mSv and 1–2 mSv) were assumed to be 0.5 mSv and 1.5 mSv, and that of the ≥2 mSv group to be 2.57 mSv, a weighted average dose by the number of residents of 0–19 years old at exposure [1]. Relative risk RR compared to the extrapolated risk at zero-dose in the 2nd round increased linearly to mean individual external dose with slope coefficient ERR/mSv of 1.43 (95%CI: 0.87, 1.99) under 2.5 mSv range (Figure 1A). To see a rough response of RR to thyroid dose estimated in UNSCEAR 2020/2021, the individual external dose was converted to thyroid dose by the linear regression formula y = 0.0067x (p = 4.2 E-14) between external dose (x/mSv) and thyroid dose in the UNSCEAR 2020/2021 for 10-year-old-children (y /Gy) of 59 municipalities (Figure 1B). RR increased linearly to converted thyroid dose with ERR/Gy of 213 (95%CI: 129, 297).
Figure 1: A) Individual dose dependence of the relative risk of thyroid cancer for three individual external dose groups <1 mSv, 1-2 mSv, and ≥2 mSv, where the external dose was converted to thyroid dose estimated in the UNSCEAR 2020/2021 report by the linear relation y= 0.0067 x. B) Thyroid dose estimated in UNSCEAR 2020/2021 for 10-year-old children versus external dose estimated by FHMS for 59 municipalities in Fukushima prefecture.
II Thyroid Cancer Incidence Rate in Japan Before and After the Fukushima Nuclear Accident
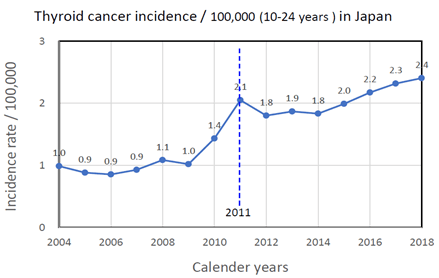
Average values of thyroid cancer incidence rate / 100,000 for young groups aged 10-24 years at diagnosis based on Japan Cancer Registries were plotted from 2004 to 2018 in (Figure 2) [15]. A sharp increase in cancer incidence rate was observed before to after 2011, the year of the nuclear accident.
Figure 2: Change of thyroid cancer incidence rate /100,000 for people aged 10-24 years at diagnosis in Japan from 2004 to 2018.
III Age Distribution of Thyroid Cancer in Fukushima
Number of thyroid cancer cases after the Chernobyl accident in Belarus, Ukraine and three contaminated oblasts in the Russian federation in 10 years after the Chernobyl accident from the UNSCEAR 2008 report and that in Fukushima for 9 years after the accident is shown in (Figure 3) ([1], Table D11 [16]).
Figure 3: Number of thyroid cancer cases in Belarus, Ukraine, and Russia in 10 years after the Chernobyl accident and in Fukushima in 9 years after the accident versus age at exposure.
Discussion
The high response of thyroid cancer incidence in the 2nd-round screening to the individual external dose suggested that the increased incidence of thyroid cancer was due to radiation exposure from the nuclear accident. This is the first finding of the linear relation between childhood thyroid cancer incidence and individual dose after the Fukushima nuclear accident. The RR of thyroid cancer incidence of dose groups increased proportionally to the mean individual external dose with ERR/mSv of 1.43 (95%CI: 0.87, 1.99) under 2.5 mSv range. Strong dose-dependence in the low external dose range and the resultant high ERR/mSv contradicts the conclusion of FHMS that radiation doses estimated so far are unlikely to cause adverse effects on health since previous epidemiological studies indicated no significant health effects at doses of ≤100 mSv [1, 2]. Based on this opinion, the Japanese government is promoting the return of residents to areas contaminated by less than 20 mSv per year.
The RR of thyroid cancer of individual dose groups increased proportionally to converted thyroid dose (y = 0.0067x where x is external dose) with ERR/Gy of 213 (95%CI: 129, 297) (Figure 1A). A rough estimate of ERR/Gy of 213 observed in the individual-dose response of thyroid cancer incidence in Fukushima was about 10-40 times higher than ERR/Gy of 5.25-23 from studies of childhood exposure to radioactive iodine during 5-14 years after the Chernobyl accident [13, 17, 18]. One reason for the high ERR/Gy might be that the thyroid dose estimates by UNSCEAR 2020/2021 were too low.
The UNSCEAR 2020/2021 concluded that the large increase of thyroid cancers detected among exposed children was the result of ultrasensitive screening and consequential overdiagnosis that revealed the prevalence of thyroid cancers in the population not previously recognized (268(q) in [4]). The definition of overdiagnosis by UNSCEAR 2020/2021 was the diagnosis that would not go on to cause symptoms or death. Thyroid cancer incidence rates in the 2nd round were tens of times higher than the rates estimated from cancer registries [3]. High incidence rates in the 2nd round after cancer cases were thoroughly detected in the 1st round indicating that the increased thyroid cancer was not the result of ultrasound screening and consequential overdiagnosis but was due to the radiation exposure from the nuclear accident. The postoperative pathological diagnoses in FMU hospital revealed that 89% of the 125 cases showed lymph node metastasis and extrathyroidal infiltration including lung metastasis, and no evidence of overdiagnosis [19].
A sharp increase in cancer incidence rate for young people aged 10-24 years at diagnosis in Japan from the average incidence rate of 1.03/100,000 in 2005-2010 to the average incidence of 2.06/100,000 after the accident (2011-2018) suggested that thyroid cancer incidence increased in Fukushima and other contaminated prefectures in eastern Japan by radiation exposure from the nuclear accident.
UNSCEAR 2020/2021 considered that the excess of thyroid cancers was probably unrelated to radiation exposure because of the difference in age distributions of thyroid cancers by age at exposure in Fukushima and the average distribution of three countries in Chernobyl (figure XXI, 226(c) in [4]). However, the number of thyroid cancer cases in 10 years after the Chernobyl accident and that in Fukushima in 9 years showed that thyroid cancer incidence was highest in the highest age group (15-18) at exposure in Fukushima, Ukraine, and Russia, while cancer cases were highest in lowest age group (0-4) only in Belarus (Figure 3). It was not correct that UNSCEAR judged the effect of radiation exposure by the age distribution of thyroid cancer.
Age distribution of thyroid cancer cases in Fukushima by age at exposure shifted to younger age, from average age (min.-max) of 14.9 (6-18) in the 1st round to 9.9 (0-18) in the 4th round + age 25 milestone screening (FY2017-2021) [1, 20]. Radiation-related thyroid cancer occurs mostly in children and adolescents, but naturally-occurring thyroid cancer incidence increases gradually with age to the highest incidence at about 65 years of age. The low age shift of thyroid cancer incidence observed in Fukushima as in Ukraine and Russia suggested the radiation origin of thyroid cancer. It should be noted that the number of childhood thyroid cancer patients in Fukushima prefecture was about a third of those in the whole country of Belarus and Ukraine. This suggests that the number of all radiation related thyroid cancer patients in Japan after the nuclear accident might have been comparable to that in Belarus and Ukraine after the Chernobyl accident.
Conclusion
The present study of thyroid cancer in Fukushima residents exposed at ≤18 years of age demonstrated a linear relationship between thyroid cancer incidence and individual external dose with ERR/mSv of 1.43 (95%CI: 0.87, 1.99) under 2.5 mSv range. This was converted to ERR/Gy of 213 (95%CI: 129, 297) (p=0.02) for thyroid dose of 10-year-old children estimated in UNSCEAR 2020/2021. This value was more than 10 times higher than ERR/Gy from studies of childhood exposure to radioactive iodine after the Chernobyl accident. Increased childhood thyroid cancer in Fukushima was found to come dominantly from radiation exposure from the nuclear accident. Observed low age shift of thyroid cancer incidence, as was observed in Ukraine and Russia after the Chernobyl accident, supported the radiation origin of thyroid cancer. A sharp increase in cancer incidence rate observed after 2011 for young people aged 10-24 years at diagnosis in Japan suggested that thyroid cancer incidence increased in contaminated prefectures in eastern Japan by radiation exposure from the nuclear accident.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 02, Apr 2022Accepted: Mon 18, Apr 2022
Published: Wed 04, May 2022
Copyright
© 2023 Toshiko Kato. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2022.02.02
Author Info
Corresponding Author
Toshiko KatoIndependent Researcher, Nara, Japan
Figures & Tables
Table
1:
Individual external dose group, mean external dose, converted thyroid dose,
thyroid cancer and non-thyroid cancer cases. Incidence, incidence odds ratio,
and relative risk compared to the extrapolated risk at zero-dose in the
second-round screening.
|
Individual external
Dose Group |
Mean external dose mSv |
Converted thyroid
dose Gy |
Thyroid cancer |
Non thyroid
cancer |
Total examinee |
Incidence / 104 |
Incidence Odds
ratio (95%CI) |
Relative risk
(95%CI) |
|
≥ 2 mSv |
2.57 |
0.0197 |
5 |
7803 |
7808 |
6.40 |
2.7 (1.0, 7.4) |
4.7 (1.7, 12.9) |
|
1~2mSv |
1.5 |
0.0115 |
16 |
37953 |
37969 |
4.21 |
1.8 (0.9, 3.6) |
3.1 (1.5, 6.3) |
|
< 1 mSv |
0.5 |
0.0038 |
15 |
63188 |
63203 |
2.37 |
1(Ref.) |
1.7 (0.9, 3.6) |
|
0 mSv |
0 |
0 |
|
|
|
|
|
1 (Ref.) |
|
Sum/Average |
|
|
36 |
108944 |
108980 |
3.30 |
|


References
1.
Radiation Medical Science Center for the Fukushima
Health Management Survey, Materials and Minutes of Prefectural Oversight
Committee Meetings.
https://fhms.jp/en/fhms/thyroid/ https://fhms.jp/en/fhms/outline/materials/
2.
Radiation Medical Science Center for the Fukushima
Health Management Survey, Fukushima Medical University, Report of the Fukushima
Health Management Survey (2019). https://fukushima-mimamori.jp/outline/uploads/report_r1.pdf
3.
Fukushima Prefectural Oversight Committee Meeting for
FHMS, Summary of the results of Full-scale Screening (Second Examination) 2019 (in Japanese).
https://www.pref.fukushima.lg.jp/uploaded/attachment/336455.pdf
4.
United Nations Scientific Committee on the Effects of
Atomic Radiation, Sources, Effects and Risks of Ionizing Radiation UNSCEAR
2020/2021 Report, Scientific Annex B.
5.
United Nations Scientific Committee on the Effects of
Atomic Radiation, UNSCEAR 2013 Report, Absorbed Dose in Thyroid in Japan for
the first year, Estimated doses to evacuees in Japan for the first year.
6.
Kato T (2019) Re: Associations between childhood
thyroid cancer and external radiation dose after the Fukushima Daiichi Nuclear
Power Plant Accident. Epidemiology 30: e9-e11. [Crossref]
7.
Kato T (2019) Area Dose Response of Prevalent
Childhood Thyroid Cancers after the Fukushima Nuclear Power Plant Accident. Clin
Oncol Res 2: 1-7.
http://dx.doi.org/10.31487/j.COR.2019.06.16
8.
Yamamoto H, Hayashi K, Scherb H (2019) Association
between the detection rate of thyroid cancer and the external radiation
dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine
(Baltimore) 98: e17165. [Crossref]
9.
Toki H, Wada T, Manabe Y, Hirota S, Higuchi T et al.
(2020) Relationship between environmental radiation and radioactivity and
childhood thyroid cancer found in Fukushima health management survey. Sci
Rep 10: 4047. [Crossref]
10. Ohira T, Ohtsuru A, Midorikawa S, Takahashi H, Yasumura S et al. (2019)
External radiation dose, obesity, and risk of childhood thyroid cancer after
the Fukushima Daiichi Nuclear Power Plant accident: The Fukushima Health
Management Survey. Epidemiology 30: 853-860. [Crossref]
11. Ohira T, Shimura H, Hayashi F, Nagao M, Yasumura S et al. (2020) Absorbed
radiation doses in the thyroid as estimated by UNSCEAR and subsequent risk of
childhood thyroid cancer following the Great East Japan Earthquake. J Radiat
Res 61: 243-248. [Crossref]
12. United Nations Scientific Committee on the Effects of Atomic Radiation
Attachment A-14, Estimates of absorbed dose to thyroid of people in Japan for
the first year after the accident at the Fukishima Daiichi Nuclear Power
Station. Attachment A-18, Estimates of effective dose and absorbed dose to the
thyroid of evacuees in Japan for the first year after the accident at the
Fukushima Daiichi Nuclear Power Station.
13. Jacob P, Kenigsberg Y, Zvonova I, Goulko G, Buglova
E et al. (1999) Childhood exposure due to the Chernobyl
accident and thyroid cancer risk in contaminated areas of Belarus and Russia. Br
J Cancer 80: 1461-1469. [Crossref]
14. Brenner AV, Tronko MD, Hatch M, Bogdanova TI,
Oliynik VA et al. (2011) I-131 dose response for incident thyroid
cancers in Ukraine related to the Chornobyl accident. Environ Health
Perspect 119: 933-939. [Crossref]
15. Cancer statistics in Japan, National Cancer Registries in Japan (1975-2015),
(2016-2018).
https://ganjoho.jp/reg_stat/statistics/data/dl/en.html
16. United Nations Scientific Committee on the Effects of Atomic Radiation,
UNSCEAR 2008 Report to the General Assembly with Scientific Annex C, D and E.
17. Jacob P, Bogdanova TI, Buglova E, Chepurniy M, Demidchik
Y, Gavrilin Y, et al. (2006) Thyroid cancer risk in areas of Ukraine and
Belarus affected by the Chernobyl accident. Radiat Res 165: 1-8. [Crossref]
18. Tronko MD, Howe RW, Bogdanova TI, Bouville AC,
Epstein OV et al. (2006) A cohort study of thyroid cancer and other
thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine
detected during the first screening. J Natl Cancer Inst 98: 897-903. [Crossref]
19. Suzuki S (2018) Surgical indication of thyroid cancer found in thyroid ultrasound examination. J Japanese Society Endocrinol Thyroid Surgery 35: 70-76. https://www.jstage.jst.go.jp/article/jaesjsts/35/2/35_70/_pdf/-char/ja
20. Kato T (2020) Change of Age Distribution of Childhood and Adolescent Thyroid Cancer after the Fukushima Nuclear Accident Compared with the Chernobyl Cases. Clin Oncol Res Volume 3: 2-5. doi:10.31487/j.COR.2020.08.20
