Impact of Vasopressor Initiation and Discontinuation Sequence on Mortality in Septic Shock: A Retrospective Review
A B S T R A C T
Background: Little data exists guiding clinicians on how or when to initiate and discontinue the second vasoactive agent in the setting of septic shock refractory to norepinephrine monotherapy.
Methods: This retrospective cohort study evaluated patients with a primary diagnosis of septic shock admitted to the intensive care unit receiving norepinephrine in addition to concomitant vasopressors. The primary endpoint was the incidence of all-cause in-hospital mortality when adding adjunctive vasopressors to norepinephrine either before the dose reached 2 mcg/kg/min (early adjunctive vasopressor) or after (late adjunctive vasopressor). Secondary endpoints included the incidence of clinically significant hypotension when discontinuing norepinephrine before or after vasopressin in the same population.
Results: Forty-six patients were included (early adjunctive vasopressor [n=36]; late adjunctive vasopressor [n=10]), with a median age of 69 years and APACHE II score of 27. Fewer patients in the early adjunctive vasopressor cohort had malignancy prior to admission (16.7% vs. 60%, p=0.0117), however, more patients were managed in the surgical ICU (44.4% vs. 0%, p=0.0202) with intra-abdominal infection (33.3% vs. 0%, p=0.0439). The primary endpoint of all-cause in-hospital mortality was not statistically different between the early and late adjunctive vasopressor groups (75% vs. 90%, respectively, p=0.4203). Longer ICU and hospital length of stay in the early adjunctive vasopressor cohort was observed (9 days vs 3 days, p=0.0061; 11 days vs 3 days, p=0.0026, respectively). Twenty-two patients were included in analysis of vasopressor discontinuation sequence with no significant differences in mortality, incidence of hypotension, or ICU/hospital length of stay.
Conclusion: Among patients with septic shock on multiple vasopressors, addition of adjunctive vasopressor before reaching a norepinephrine dose of 2 mcg/kg/min was associated with longer in-hospital and ICU survival but exhibited no difference in overall mortality. Discontinuation of vasopressin before norepinephrine led to longer total vasopressor duration without a difference in rates of hypotension. Future prospective studies are warranted.
Keywords
Sepsis, septic shock, vasopressors, norepinephrine, vasopressin
Introduction
The 2016 Surviving Sepsis Campaign define sepsis as the life-threatening organ dysfunction induced by a dysregulated host response to infection that quickly degenerate into significant vasoplegia and shock [1, 2]. Given the advancements in research and efforts for early recognition, septic shock remains one of the most common diagnoses made for the critically ill patient being treated in the intensive care unit (ICU), with greater than 750,000 cases in the United States each year [1]. However, despite the developments made in understanding and treatment, mortality associated with septic shock remains exceedingly highly, often greater than 50% [2]. Specifically, Hemodynamic support is often required due to profound vasodilation and capillary leak secondary to the release of inflammatory mediators [2, 3]. Current guidelines for the management of septic shock suggest maintaining a mean arterial pressure (MAP) of 65 mmHg in order to maintain end-organ perfusion [2]. Thus, vasopressor therapy is often required when adequate fluid resuscitation is not sufficient, defining the onset of septic shock.
In patients with profound shock refractory to mono-vasopressor therapy, a second vaso-active agent is required, however, the optimal agent has been debated in the literature [4]. The consensus in the literature and the recommendations from the Surviving Sepsis Campaign include the primary initiation of norepinephrine for initial septic shock management, however, the recommendations for subsequent therapy remain unclear [2]. Epinephrine remains high utilized given its initial inclusion in the original Surviving Sepsis guideline, and the use of vasopressin has also been granted a weak recommendation based on low quality evidence. Though the clinical evidence in randomized trials remains weak, vasopressin has shown to decrease vasopressor requirements with the assumption of relative vasopressin deficiency seen during septic shock [2, 5]. Further, limited guidance exists for when to initiate adjunctive vasopressin therapy, though some suggest better outcomes when adding to lower doses of norepinephrine or earlier in norepinephrine therapy [6].
Data regarding the recovery phase of septic shock is also scarce with no specific consensus recommendations in the literature. Patients can continue to experience clinically significant hypotension after vasopressor discontinuation which has been associated with poor outcomes [5-6]. Thus, the optimal approach to discontinuing vasopressors, especially for those patients that required multiple agents has been of question, with several hypotheses that continuing vasopressin therapy and discontinuing norepinephrine first may be beneficial in the likely setting of vasopressin deficiency that can persist for 36 to 96 hours [5, 7].
Given the sparsity of evidence and definitive recommendations in the literature for appropriate initiation and discontinuation of vasopressors in septic shock, this study aimed to characterize the timing of adjunctive vasopressor initiation and discontinuation and its impact on mortality in patients with septic shock. Specifically, we hypothesized that patients with septic shock who were initiated on adjunctive vasopressor before reaching 2 mcg/kg/min of norepinephrine would have increased rates of survival to hospital discharge. Subsequently, in patients recovering from septic shock, discontinuation of vasopressin before norepinephrine will result in more frequent development of clinically significant hypotension (MAP < 65 mmHg) and mortality.
Methods
I Study Design, Setting, Patient Population
This investigation was a retrospective cohort analysis at a single, tertiary-care, urban, 957-bed academic medical center in the United States approved by an Institutional Review Board, who waived the need for informed consent. Data was extracted from the electronic medical record for all consecutive patients 18 years of age or older admitted between July 2017 and July 2018 with a primary admission diagnosis of septic shock, as evidenced by ICD-10 codes A419, R6520, and R6521, and a charge for norepinephrine administration. In order to meet eligibility criteria, patients must have been initiated on two or more vasopressors, one of which must have been norepinephrine, for the management of septic shock.
Patients were excluded if vasopressors were being prescribed for an alternative indication, vasopressor use lasted less than 12 consecutive hours, or there was no documentation in the chart regarding accurate vasopressor initiation and discontinuation times (including patients transferred from an outside hospital with inaccessible records). Included patients were then stratified based upon the dose of norepinephrine at which the first adjunctive vasopressor was added. The early group was defined as the first adjunctive vasopressor added at a norepinephrine dose <2mcg/kg/min. Whereas the late group was defined as the first adjunctive vasopressor added at a norepinephrine dose ≥ 2mcg/kg/min. Secondarily, a subgroup of patients that survived their initial septic shock, were managed with vasopressin as their first adjunctive vasopressor and entered the recovery phase were stratified based on which vasopressor was discontinued first, either norepinephrine or vasopressin.
II Outcomes
The primary outcome evaluated was the incidence of all-cause in-hospital mortality, reported as the percentage of patients in the early compared to late adjunctive vasopressor group. ICU and hospital length of stay were evaluated as surrogate measures of duration of survival. Secondary outcomes included percentage of patients who experienced clinically significant hypotension [defined by MAP <65 mmHg, systolic blood pressure <90 mmHg, or change in systolic blood pressure by ≥40 mmHg] and mortality after the discontinuation of the adjunctive vasopressor prior to discontinuation of norepinephrine.
III Data Analysis
Data regarding patient demographics, including age, sex, weight, primary infection, medication profiles, and corresponding lab values were collected from the electronic medical record. Data was handled and de-identified by the hospital pharmacy staff. All data was then analyzed using descriptive statistics, including mean, median, mode, and standard deviation. Binary outcomes were evaluated using chi-square (or Fischer’s exact for small sample sizes) and continuous data were analyzed using independent sample student’s t-test. A two-tailed α of 0.05 was assumed for analysis of statistical significance.
Results
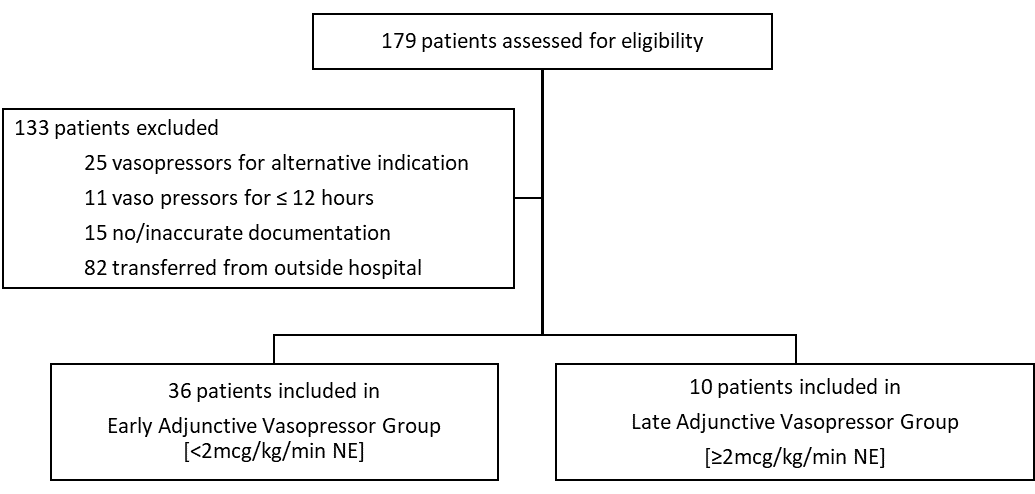
During the 12-month study period, 179 patients who were coded with a primary diagnosis of septic shock were screened for inclusion (Figure 1). One hundred and thirty-three (133) patients were excluded secondary to alternative indication for vasopressor therapy (25 patients), vasopressor duration less than 12 hours (11 patients), inaccurate documentation in the medication administration record (15 patients), unable to assess outside hospital record (82 patients). This left 46 patients who were the included in the final statistical analysis. Of the 46 patients included, 36 received adjunctive vasopressor before reaching a norepinephrine dose of 2 mcg/kg/min (early adjunctive vasopressor group) and 10 patients received adjunctive vasopressor once the norepinephrine dose was equal to 2 mcg/kg/min (late adjunctive vasopressor group). Table 1 lists the baseline patient demographics of each group.
Figure 1: Patient Selection.
Table 1: Baseline Demographics.
|
|
Early (n=36) |
Late (n=10) |
P-value |
|
Age – years, median (SD) |
70.5 (9.6) |
65.5 (9.7) |
0.9445 |
|
Male – n (%) |
20 (55.5) |
5 (50) |
0.7322 |
|
Race – n (%) |
|
|
|
|
White |
24 (66.6) |
3 (30) |
0.0672 |
|
Black |
7 (19.4) |
7 (70) |
0.0045 |
|
Other |
5 (14) |
0 |
0.3359 |
|
Weight – kilograms, median (SD) |
78.2 (25.5) |
63.6 (18.8) |
0.3754 |
|
BMI – kg/m2, median (SD) |
26.5 (8.3) |
25 (6.5) |
0.2352 |
|
Past medical history – n (%) |
|
|
|
|
Chronic Kidney Disease |
8 (22.2) |
2 (20) |
0.9999 |
|
Congestive Heart Failure |
8 (22.2) |
1 (10) |
0.6590 |
|
Diabetes Mellitus |
13 (36.1) |
2 (20) |
0.4599 |
|
Hypertension |
21 (58.3) |
5 (50) |
0.7264 |
|
Immunosuppression |
6 (16.7) |
0 (0) |
0.3145 |
|
Liver Disease |
11 (30.5) |
1 (10) |
0.2524 |
|
Malignancy |
6 (16.7) |
6 (60) |
0.0117 |
|
Intensive Care Unit – n (%) Medical Surgical/Trauma Surgical/Cardiothoracic Cardiac Neurosciences/Neurosurgery |
14 (39) 16 (44.4) 0 (0) 3 (8.3) 3 (8.3) |
10 (100) 0 (0) 0 (0) 0 (0) 0 (0) |
0.0006 0.0202 0.9999 0.5850 0.5850 |
|
APACHE II score – median (IQR) |
26 (24, 32.75) |
32 (27, 43) |
0.0952 |
|
SOFA score – median (IQR) |
12.5 (8.25, 13.75) |
14 (12, 16) |
0.0192 |
Note: SD: Standard Deviation, APACHE II: Acute Physiology and Chronic Health Evaluation II, SOFA: Sequential Organ Failure Assessment, IQR: Interquartile Range.
A statistically significant difference between the early and late adjunctive vasopressor cohorts was detected between the percentage of patients that were black (19.4% vs. 70%, respectively, p=0.0045) and the percentage of patients that were diagnosed with malignancy prior to admission (16.7% vs. 60%, respectively, p=0.0117). There were more patients in the early cohort that were diagnosed with intra-abdominal sources of infection (33.3% vs. 0%, p=0.0439) and were being cared for in the surgical/trauma ICU (44.4% vs. 0%, p=0.0202). All patients in the late group were being cared for in the medical ICU. The median Acute Physiology and Chronic Health Evaluation (APACHE) II score was numerically lower in the early cohort, though this was not statistically significant (26 vs. 32, p=0.0952); however, the SOFA score upon vasopressor initiation was statistically lower in the early cohort (12.5 vs. 14, p=0.0192).
Patient characteristics at the time of vasopressor initiation are described in (Table 2). Selection of adjunctive vasopressor therapy for septic shock were similar between groups, with a majority of patients receiving vasopressin. The median norepinephrine dose was 0.875 mcg/kg/min at which adjunctive vasopressors was added in the early group. All patients in the late group received adjunctive vasopressors after the norepinephrine dose reached 2mcg/kg/min. The median fluid bolus was numerically higher in the early cohort (41.6 mL/kg vs. 35.3 mL/kg, respectively, p=0.6534) along with the median MAP at which the adjunctive vasopressor was added (69 mmHg vs. 59.65 mmHg, p=0.1475), but neither reached statistical significance. Zero patients in either group were started on epinephrine as the first adjunctive vasopressor, however it was third- or fourth-line therapy in nine patients in the early group (25%) and four patients in the late group (40%). Medications, including sedation and inotropes, which may have negatively impacted vascular tone, that were administer adjunctively in each study group are listed in (Table 2), with no significant difference in usage between groups.
Table 2: Patient Characteristics at time of Vasopressor Initiation.
|
|
Early (n=36) |
Late (n=10) |
P-value |
|
Site of infection – n (%) Pulmonary Intra-abdominal Urinary tract Bone/Joint Cardiac/Endocarditis Line/Bloodstream Skin and soft tissue Other |
12 (33.3) 12 (33.3) 2 (5.6) 0 (0) 0 (0) 2 (5.6) 0 (0) 8 (22.2) |
7 (70) 0 (0) 0 (0) 0 (0) 0 (0) 2 (20) 0 (0) 1 (10) |
0.0672 0.0439 0.9999 0.9999 0.9999 0.2015 0.9999 0.6590 |
|
Need for MV during course of vasopressor therapy – n (%) |
33 (91.6) |
10 (100) |
0.5489 |
|
Need for RRT during course of vasopressor therapy – n (%) |
21 (58.3) |
6 (60) |
0.9999 |
|
Concomitant medications during course of vasopressor therapy – n (%) Ascorbic acid, thiamine, hydrocortisone Hydrocortisone monotherapy Midodrine Intravenous sodium bicarbonate Dobutamine Milrinone Propofol Midazolam |
21 (58.3)
6 (16.6) 4 (11.1) 6 (16.7) 12 (33.3) 4 (11.1) 2 (5.6) 7 (47.2) 6 (16.7) |
4 (40)
3 (30) 3 (30) 0 (0) 7 (70) 1 (10) 0 (0) 3 (30) 3 (30) |
0.4748
|
|
Fluids administered prior to vasopressor therapy – mL/kg, median (SD) |
41.6 (23.2) |
35.3 (11.9) |
0.6534 |
|
First adjunctive vasopressor initiated – n (%) Phenylephrine Vasopressin |
3 (8.3) 33 (91.6) |
1 (10) 9 (90) |
0.9999
|
|
Norepinephrine dose at time of first adjunctive vasopressor initiation – mcg/kg/min, median (IQR) |
0.875 (0.375, 1.25) |
2 (2, 2) |
<0.001 |
|
-- mcg/min |
55.3175 |
127.2 |
<0.001 |
|
MAP at the time of adjunctive vasopressor initiation – mmHg, median (SD) |
69 (13.6) |
59.5 (11.2) |
0.1475 |
|
Median total number of concomitant vasopressors |
3 |
3 |
0.0695 |
Note: SD: Standard Deviation, MV: Mechanical Ventilation, RRT: Renal Replacement Therapy, MAP: Mean Arterial Pressure, IQR: Interquartile Range.
I Outcomes
Comparisons of the early compared to late adjunctive vasopressor cohorts in terms of the primary outcomes of all-cause in-hospital mortality, along with the surrogate measures of survival, including ICU and hospital length of stay are described in (Table 3). The primary endpoint of all-cause in-hospital mortality was not statistically different between the early and late adjunctive vasopressor groups (75% vs. 90%, respectively, p=0.4203). A majority of patients in the late adjunctive vasopressor cohort expired in the ICU, resulting in a shorter ICU and hospital length of stay when compared to patients in the early group (9 days vs. 3 days, p=0.0061; 11 days vs. 3 days, p=0.0026, respectively).
Given the high rate of in-hospital mortality, analysis of survivors to hospital discharge was also performed in regard to several of the secondary endpoints (Table 4). Of the 46 patients included in the analysis, 10 patients survived to hospital discharge, nine patients in the early group and 1 patient in the late group. The survivors in the early group had longer median ICU and hospital length of stay (23 days vs. 13 days, 30 days vs. 15 days, respectively), that was consistent with previously mentioned analysis of the entire cohort.
Table 3: Outcomes Based Upon Timing of the First Adjunctive Vasopressor.
|
|
Early (n=36) |
Late (n=10) |
P-value |
|
In-hospital mortality – n (%) |
27 (75) |
9 (90) |
0.4203 |
|
ICU length of stay – days, median (IQR) |
9 (4.75, 21.25) |
3 (1, 6.25) |
0.0061 |
|
Hospital length of stay – days, median (IQR) |
11 (8, 24) |
3 (1, 6.25) |
0.0026 |
|
Total duration of norepinephrine – hours, median (IQR) |
96.5 (40.2, 167.1) |
70.875 (25.25, 133.875) |
0.3240 |
|
Total duration of adjunctive vasopressor – hours, median (IQR) |
26 (14.4, 49.5) |
17 (14, 20.25) |
0.3413 |
Note: SD: standard deviation, ICU: intensive care unit, IQR: interquartile range.
Table 4: Outcomes of Survival Analysis.
|
|
Early (n=9) |
Late (n=1) |
|
ICU length of stay – days, median (IQR) |
23 (11, 28) |
13 |
|
Hospital length of stay – days, median (IQR) |
30 (16, 62) |
15 |
|
Total duration of norepinephrine – hours, median (IQR) |
104 (66, 134.75) |
57.25 |
|
Total duration of adjunctive vasopressor – hours, median (IQR) |
48.5 (27, 68.75) |
73.5 |
Note: SD: Standard Deviation, ICU: Intensive Care Unit, IQR : Interquartile Range.
Twenty-one of the 46 patients expired while on multiple vasopressor therapy and three patients were adjunctively on phenylephrine as their second vasopressor, leaving 22 patients, who survived their initial septic shock and entered recovery phase, to be included for analysis of secondary outcome measures (Figure 2). As listed in (Table 5), three patients were weaned off norepinephrine before vasopressin and 19 patients were weaned off vasopressin before norepinephrine. The group of patients in which norepinephrine was discontinued first (continued on vasopressin for the duration of their shock state) experienced a significantly shorter duration of total vasopressor (88 hours vs. 182.5 hours, p=0.0018). Additionally, patients in which norepinephrine was discontinued first also had numerically less incidence of clinically significant hypotension (33.3% vs 78.5%) and less in-hospital mortality (0% vs 63.2%), but this data did not reach statistical significance. Otherwise, there were no significant differences between the two groups, including ICU or hospital length of stay.
Table 5: Norepinephrine and Vasopressin Discontinuation Sequence Outcomes.
|
|
Norepinephrine first (n=3) |
Vasopressin first (n=19) |
P-value |
|
Incidence of clinically significant hypotension – n (%) |
1 (33.3) |
15 (78.9) |
0.1688 |
|
In-hospital mortality – n (%) |
0 (0) |
12 (63.2) |
0.0779 |
|
ICU length of stay – days, median (IQR) |
14 (13.5, 30.5) |
12 (8.5, 22.5) |
0.5530 |
|
Hospital length of stay – days, median |
16 (15.5, 34) |
20 (9.5, 28) |
0.9708 |
|
Total duration of norepinephrine – hours, median (IQR) |
66 (61.25, 68) |
173 (114.1, 258.5) |
<0.001 |
|
Total duration of vasopressin – hours, median (IQR) |
67 (44.5, 70.25) |
34 (2, 51.6) |
0.4654 |
|
Total duration of vasopressors – hours, median (IQR) |
88 (77.9, 90) |
182.5 (127.25, 279.75) |
0.0018 |
Note: SD: Standard Deviation, ICU: Intensive Care Unit, IQR: Interquartile Range.
Figure 2: Discontinuation Sequence Outcomes.
Discussion
Limited data evaluating the sequence by which clinicians add and discontinue vasopressors in septic shock exists. Though the most recent iteration of the “Surviving Sepsis Campaign” suggest the addition of epinephrine or vasopressin to norepinephrine, very limited and weak evidence suggests how and when to initiate adjunctive vasopressors [2]. Our study evaluated both of these questions in a single patient population, including a subset of patients who entered septic shock recovery. Through our investigation, we were not able to show significant differences in all-cause in-hospital mortality but did find significant differences in terms of hospital and ICU length of stay. Our study showed that patients that were initiated on adjunctive vasopressor at lower norepinephrine survived longer in the hospital, which may allude to longer survival overall, though we were not adequately powered to show this difference.
Russell et al illustrated in a large, prospective, randomized trial also addressed this issue and showed that the addition of vasopressin to norepinephrine in patients with septic shock exhibited no differences in 28-day (35.4% vs 39.3%, p=0.26) or 90-day mortality (43.9 vs 49.6%, p=0.11) when compared to norepinephrine alone [6]. However, the study demonstrated improved survival among patients with less severe septic shock, as defined by those patients requiring less than 15 mcg/min of norepinephrine [6]. Though the investigators found a difference in their a priori subgroup analysis of less severe septic shock, it still remains uncertain on when to initiate adjunctive therapy and the overall mechanism by which benefit is derived. In our retrospective study, we discovered similar findings in regard to mortality; however, our results trended toward lower incidence in patients in which adjunctive vasopressors were added to lower norepinephrine doses.
In regard to vasopressor discontinuation strategies, our study demonstrated that discontinuation of norepinephrine before vasopressin resulted in shorter total duration of vasopressor therapy with trends toward less clinically significant hypotension. Though vasopressin has only shown modest improvements in MAP and reductions in norepinephrine dose requirements it has long been believed that vasopressin levels in septic shock are reportedly lower than anticipated [2, 6, 8]. In the acute hyper-dynamic phase of sepsis, vasopressin concentrations are elevated but then subsequently decrease to lower than expected levels by 24 to 48 hours after shock onset [9]. There is believed to be a relative vasopressin deficiency as septic shock progresses, data regarding administration of vasopressin through the recovery phase of shock has been controversial. Bauer et al assessed this issue by studying and assessing the incidence of hypotension when vasopressin is removed prior to norepinephrine in a retrospective review of 50 patients with septic shock [10]. The study investigators discovered that discontinuation of vasopressin prior to norepinephrine in the recovering septic shock state led to a higher and quicker incidence of hypotension [10].
These findings were reproduced by Musallam et al, in which they noted a 7-fold increased risk in hypotension when vasopressin was discontinued first [7]. While the findings of our review did not substantiate these claims, we discovered a longer duration of vasopressor therapy in the cohort of patients in which vasopressin was discontinued first, which may correlate with relative vasopressin deficiency in these patients leading to prolonged hypotension. In relation to previously published trials and analyses, our study has several notable differences. Specifically, we evaluated and stratified patients via a weight-based dose of norepinephrine in terms of the management of their septic shock, which provided a broader assessment of dosing range on clinical outcomes compared to previous studies. Further, we then assessed the notion of relative vasopressin insufficiency via the analysis of clinically relevant hypotension once patients reached the recovery phase of septic shock through the duration of their hospital stay, which may account for patient specific variations in vasopressin deficiency and recovery as opposed to the immediate post-vasopressin discontinuation phase that is examined in most other studies. Finally, we included patients of varying illness severities, as evidenced by higher than typically reported APACHE II and SOFA scores, which allowed us to assess the clinical implications of vasopressor initiation and discontinuation in very sick patients, enhancing our generalizability.
Several limitations should be assessed in the interpretation of our results. First, this analysis was performed at a single center in a retrospective manner with a very limited sample size. Given the small number of patients included for analysis, our study may have been underpowered to detect a true difference in our primary outcome of in-hospital mortality. Second, vasopressor initiation and discontinuation protocols were not standardized at our institution, leading to provider specific practices that may have influenced the study population. None of our cohort of patients received epinephrine as their first adjunctive vasopressor, which is the current guideline recommendation, which may also affect the generalizability and applicability of our practices. Additionally, due to the retrospective design, it was difficult to ascertain the provider’s intent regarding the rationale behind vasopressor choice. Finally, there were several heterogeneities in our patient population that may have confounded the outcome. Most notably, the high median APACHE II and SOFA scores along with the disproportionate number of patients with malignancy in each group may have skewed our overall findings and biased our mortality outcomes.
Conclusion
Little evidence exists to guide practitioners on how to initiate adjunctive vasoactive medications in addition to norepinephrine in patients with refractory septic shock. Moreover, once shock has begun to resolve there is much ambiguity in the literature on which agent to discontinue first, norepinephrine or vasopressin. This study suggests that addition of adjunctive vasopressors, specifically vasopressin, prior to maximal norepinephrine dose of 2mcg/kg/min may prolong survival. Additionally, discontinuation of vasopressin prior to norepinephrine showed longer total vasopressor time but did not suggest differences in negative outcomes. Prospective, randomized, controlled trials are warranted to better elucidate causality and confirm these findings.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 30, Dec 2019Accepted: Thu 13, Feb 2020
Published: Fri 21, Feb 2020
Copyright
© 2023 Cara McDaniel. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.01.05
Author Info
Andrew Moyer Cara McDaniel Judah Brown Michael Baram
Corresponding Author
Cara McDanielDepartment of Pharmacy, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA
Figures & Tables
Table 1: Baseline Demographics.
|
|
Early (n=36) |
Late (n=10) |
P-value |
|
Age – years, median (SD) |
70.5 (9.6) |
65.5 (9.7) |
0.9445 |
|
Male – n (%) |
20 (55.5) |
5 (50) |
0.7322 |
|
Race – n (%) |
|
|
|
|
White |
24 (66.6) |
3 (30) |
0.0672 |
|
Black |
7 (19.4) |
7 (70) |
0.0045 |
|
Other |
5 (14) |
0 |
0.3359 |
|
Weight – kilograms, median (SD) |
78.2 (25.5) |
63.6 (18.8) |
0.3754 |
|
BMI – kg/m2, median (SD) |
26.5 (8.3) |
25 (6.5) |
0.2352 |
|
Past medical history – n (%) |
|
|
|
|
Chronic Kidney Disease |
8 (22.2) |
2 (20) |
0.9999 |
|
Congestive Heart Failure |
8 (22.2) |
1 (10) |
0.6590 |
|
Diabetes Mellitus |
13 (36.1) |
2 (20) |
0.4599 |
|
Hypertension |
21 (58.3) |
5 (50) |
0.7264 |
|
Immunosuppression |
6 (16.7) |
0 (0) |
0.3145 |
|
Liver Disease |
11 (30.5) |
1 (10) |
0.2524 |
|
Malignancy |
6 (16.7) |
6 (60) |
0.0117 |
|
Intensive Care Unit – n (%) Medical Surgical/Trauma Surgical/Cardiothoracic Cardiac Neurosciences/Neurosurgery |
14 (39) 16 (44.4) 0 (0) 3 (8.3) 3 (8.3) |
10 (100) 0 (0) 0 (0) 0 (0) 0 (0) |
0.0006 0.0202 0.9999 0.5850 0.5850 |
|
APACHE II score – median (IQR) |
26 (24, 32.75) |
32 (27, 43) |
0.0952 |
|
SOFA score – median (IQR) |
12.5 (8.25, 13.75) |
14 (12, 16) |
0.0192 |
Note: SD: Standard Deviation, APACHE II: Acute Physiology and Chronic Health Evaluation II, SOFA: Sequential Organ Failure Assessment, IQR: Interquartile Range.
Table 2: Patient Characteristics at time of Vasopressor Initiation.
|
|
Early (n=36) |
Late (n=10) |
P-value |
|
Site of infection – n (%) Pulmonary Intra-abdominal Urinary tract Bone/Joint Cardiac/Endocarditis Line/Bloodstream Skin and soft tissue Other |
12 (33.3) 12 (33.3) 2 (5.6) 0 (0) 0 (0) 2 (5.6) 0 (0) 8 (22.2) |
7 (70) 0 (0) 0 (0) 0 (0) 0 (0) 2 (20) 0 (0) 1 (10) |
0.0672 0.0439 0.9999 0.9999 0.9999 0.2015 0.9999 0.6590 |
|
Need for MV during course of vasopressor therapy – n (%) |
33 (91.6) |
10 (100) |
0.5489 |
|
Need for RRT during course of vasopressor therapy – n (%) |
21 (58.3) |
6 (60) |
0.9999 |
|
Concomitant medications during course of vasopressor therapy – n (%) Ascorbic acid, thiamine, hydrocortisone Hydrocortisone monotherapy Midodrine Intravenous sodium bicarbonate Dobutamine Milrinone Propofol Midazolam |
21 (58.3)
6 (16.6) 4 (11.1) 6 (16.7) 12 (33.3) 4 (11.1) 2 (5.6) 7 (47.2) 6 (16.7) |
4 (40)
3 (30) 3 (30) 0 (0) 7 (70) 1 (10) 0 (0) 3 (30) 3 (30) |
0.4748
|
|
Fluids administered prior to vasopressor therapy – mL/kg, median (SD) |
41.6 (23.2) |
35.3 (11.9) |
0.6534 |
|
First adjunctive vasopressor initiated – n (%) Phenylephrine Vasopressin |
3 (8.3) 33 (91.6) |
1 (10) 9 (90) |
0.9999
|
|
Norepinephrine dose at time of first adjunctive vasopressor initiation – mcg/kg/min, median (IQR) |
0.875 (0.375, 1.25) |
2 (2, 2) |
<0.001 |
|
-- mcg/min |
55.3175 |
127.2 |
<0.001 |
|
MAP at the time of adjunctive vasopressor initiation – mmHg, median (SD) |
69 (13.6) |
59.5 (11.2) |
0.1475 |
|
Median total number of concomitant vasopressors |
3 |
3 |
0.0695 |
Note: SD: Standard Deviation, MV: Mechanical Ventilation, RRT: Renal Replacement Therapy, MAP: Mean Arterial Pressure, IQR: Interquartile Range.
Table 3: Outcomes Based Upon Timing of the First Adjunctive Vasopressor.
|
|
Early (n=36) |
Late (n=10) |
P-value |
|
In-hospital mortality – n (%) |
27 (75) |
9 (90) |
0.4203 |
|
ICU length of stay – days, median (IQR) |
9 (4.75, 21.25) |
3 (1, 6.25) |
0.0061 |
|
Hospital length of stay – days, median (IQR) |
11 (8, 24) |
3 (1, 6.25) |
0.0026 |
|
Total duration of norepinephrine – hours, median (IQR) |
96.5 (40.2, 167.1) |
70.875 (25.25, 133.875) |
0.3240 |
|
Total duration of adjunctive vasopressor – hours, median (IQR) |
26 (14.4, 49.5) |
17 (14, 20.25) |
0.3413 |
Note: SD: standard deviation, ICU: intensive care unit, IQR: interquartile range.
Table 4: Outcomes of Survival Analysis.
|
|
Early (n=9) |
Late (n=1) |
|
ICU length of stay – days, median (IQR) |
23 (11, 28) |
13 |
|
Hospital length of stay – days, median (IQR) |
30 (16, 62) |
15 |
|
Total duration of norepinephrine – hours, median (IQR) |
104 (66, 134.75) |
57.25 |
|
Total duration of adjunctive vasopressor – hours, median (IQR) |
48.5 (27, 68.75) |
73.5 |
Note: SD: Standard Deviation, ICU: Intensive Care Unit, IQR : Interquartile Range.
Table 5: Norepinephrine and Vasopressin Discontinuation Sequence Outcomes.
|
|
Norepinephrine first (n=3) |
Vasopressin first (n=19) |
P-value |
|
Incidence of clinically significant hypotension – n (%) |
1 (33.3) |
15 (78.9) |
0.1688 |
|
In-hospital mortality – n (%) |
0 (0) |
12 (63.2) |
0.0779 |
|
ICU length of stay – days, median (IQR) |
14 (13.5, 30.5) |
12 (8.5, 22.5) |
0.5530 |
|
Hospital length of stay – days, median |
16 (15.5, 34) |
20 (9.5, 28) |
0.9708 |
|
Total duration of norepinephrine – hours, median (IQR) |
66 (61.25, 68) |
173 (114.1, 258.5) |
<0.001 |
|
Total duration of vasopressin – hours, median (IQR) |
67 (44.5, 70.25) |
34 (2, 51.6) |
0.4654 |
|
Total duration of vasopressors – hours, median (IQR) |
88 (77.9, 90) |
182.5 (127.25, 279.75) |
0.0018 |
Note: SD: Standard Deviation, ICU: Intensive Care Unit, IQR: Interquartile Range.

References
- Finfer S, Machado FR (2016) The Global Epidemiology of Sepsis. Does It Matter That We Know So Little? Am J Respir Crit Care Med 193: 228-230. [Crossref]
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M et al. (2017) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 43: 304-377. [Crossref]
- Singer M, Deutschman CS, Seymour CW, Shankar Hari M, Annane D et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801-810. [Crossref]
- Nagendran M, Maruthappu M, Gordon AC, Gurusamy KS (2016) Comparative safety and efficacy of vasopressors for mortality in septic shock: A network meta-analysis. J Intensive Care Soc 17: 136-145. [Crossref]
- Bissell BD, Magee C, Moran P, Bastin MLT, Flannery AH (2019) Hemodynamic Instability Secondary to Vasopressin Withdrawal in Septic Shock. J Intensive Care Med 34: 761-765. [Crossref]
- Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC et al. (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358: 877-887. [Crossref]
- Musallam N, Altshuler D, Merchan C, Zakhary B, Aberle C et al. (2018) Evaluating Vasopressor Discontinuation Strategies in Patients With Septic Shock on Concomitant Norepinephrine and Vasopressin Infusions. Ann Pharmacother 52: 733-739. [Crossref]
- Landry DW, Levin HR, Gallant EM, Ashton RC Jr, Seo S et al. (1997) Vasopressin deficiency contributes to vasodilation of septic shock. Circulation 95: 1122-1125. [Crossref]
- Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P et al. (2003) Circulating vasopressin levels in septic shock. Crit Care Med 31: 1752-1758. [Crossref]
- Bauer SR, Aloi JJ, Ahrens CL, Yeh JY, Culver DA et al. (2010) Discontinuation of vasopressin before norepinephrine increases the incidence of hypotension in patients recovering from septic shock: a retrospective cohort study. J Crit Care 25: 362. [Crossref]
