Impact of Persistent N2 Disease and Lymph Node Ratio on Oncological Outcomes after Multimodal Treatment in Pre-Operative Histologically Proven N2 Disease Non-Small-Cell Lung Cancer
A B S T R A C T
Objective: The objectives of our retrospective analysis were to estimate the oncological long-term results of patients with ypN2 and to evaluate the impact of lymph node ratio (LNR) on overall (OS) and disease-free survival (DFS).
Methods: We analysed all consecutive patients (n=85) undergoing neoadjuvant chemotherapy (NAC) and surgery for pre-operative pathologically proven stage IIIA-B (N2) NSCLC from 2014 to 2020. Median LNR (0.29 or 29%) was selected as threshold for grouping. Survival was estimated using the Kaplan-Meier method. Cox regression was used to test the association between OS, DFS and covariates.
Results: Post-operative mortality was 3.5%. The median follow up was 21 months (range 6 69 months). The 5-year OS and DFS of the cohort were 41% and 20%. Patients with LNR>0.29 (n=13; 15.3%) showed a trend toward worse survival than patients with LNR0 (n=44; 51.8%) with a 5-year OS of 56% VS 14% (p=0.077), confirmed as a trend at the multivariable analysis (HR 2.28; p=0.066). At the univariate analysis a worse DFS was observed for ypN2 patients (n=58; 68.2%) compared with nodal downstaging (46% vs 25% 3-year DFS, p=0.039). DFS was different according to LNR: 3-year DFS was 14% in patients with LNR>0.29 while it reached 44% in patients with LNR 0 (p=0.043) and 62% in LNR<0.29 (p=0.03). LNR>0.29 was the only significant predictor (HR 2.89; p=0.047) of reduced DFS at the multivariable analysis.
Conclusion: patients with ypN2 disease after NAC showed acceptable oncological outcomes and this finding is true for patients with low burden of nodal disease assessed by LNR.
Keywords
Lymph node ratio, persistent N2 disease, multimodal treatment, stage IIIA-B (N2) non-small-cell lung cancer, neoadjuvant therapy
Introduction
Multimodality treatment is currently the standard of care for resectable non-small-cell lung cancer (NSCLC) with ipsilateral mediastinal lymph node metastasis (N2 disease). In this setting, international guidelines suggest the use of chemotherapy, radiation therapy and surgery, but the combination and timing of these modalities are still matter of controversy, particularly regarding the indications to surgery, in part due to the well-known heterogeneity of the stage III (N2) NSCLC [1-4]. Some prognostic factors have been identified including the persistence of N2 disease that had a strong negative impact on overall (OS) and disease-free survival (DFS) and surgery is usually reserved for patients experiencing a mediastinal downstaging after induction therapy [5-8]. However, clinical restaging is often inaccurate leading to perform surgery in patients with persistent N2 disease, and some of these patients may benefit from surgery, even if we do not know how to identify them before the procedure [5, 7-12].
To better define the “burden of nodal disease”, a new subclassification of nodal involvement has been proposed in the latest staging system which considers both the localization and the amount of N2 disease, but it has not been widely applied into the clinical practice yet because of the difficulties of an accurate assessment during clinical staging and some authors suggested the use of the lymph node ratio (LNR) [13-18]. However, the influence of LNR on oncological outcomes after multimodal treatment is still an argument of debate [19, 20].
The objectives of our retrospective analysis were: i) to evaluate the oncological long-term results of our multimodality curative-intent approach (neoadjuvant chemotherapy -NAC- followed by surgery) in patients affected by stage IIIA-B (N2) NSCLC focusing on the outcomes of patients with persistent N2 disease (ypN2); and ii) to evaluate the impact of LNR on OS and DFS.
Patients and Methods
Patients who had lung resection after NAC for clinical stage IIIA-B (N2) NSCLC at Careggi University Hospital of Florence, were retrospectively reviewed from a prospectively maintained thoracic surgery database from June 2014 to March 2020. Our institutional review board granted approval and waived the requirement for specific informed consent for this retrospective study.
The inclusion criteria were as follows:
i. Patients with clinical stage IIIA-B (N2) NSCLC who underwent surgery after NAC.
ii. Pre-operative cyto-histologically proven N2 disease through videomediastinoscopy (VM) or endobronchial or oesophageal ultrasound (EBUS/EUS).
iii. Tumor resectability before and after NAC in absence of radiological tumor progression.
iv. Adequate cardio-respiratory reserve and Eastern Cooperative Oncology Group Performance Status (ECOG-PS) between 0 and 2.
Patients with bulky N2 disease or with N3 disease (contralateral or supraclavicular stations) before NAC and patients who underwent neoadjuvant chemoradiation therapy for involvement of the chest wall or superior sulcus tumor (n=21) were excluded. Baseline and post-operative variables included demographics, comorbidities, pulmonary and cardiovascular assessment, clinical stage, pathological stage, hospital length of stay, post-operative complications and peri-operative mortality (within 90 days or during the same hospital stay).
Clinical stage was assessed by whole-body computer tomographic scan (wb-CT), positron emission tomographic scan (PET-CT-scan), bronchoscopy, endobronchial or oesophageal ultrasound (EBUS/EUS) and videomediastinoscopy. Clinical and pathological stages were resumed with the American Joint Committee on Cancer 8th Edition TNM Classification [21]. Stages obtained before the publication of the 8th Edition were re-checked and reported by the first author (B.S.). All patients were evaluated by our institutional Multidisciplinary Tumor Board (MTB) and the individual treatment was decided based on clinical stage and the most recent international guidelines [1]. A large part of patients (96.5%) received a platinum-based regimen associated with other different drugs. After 3 to 4 courses of NAC, patients without evidence of distant or local disease progression at the restaging performed with wb-CT-scan and PET-CT-scan underwent lung resection and en-bloc ipsilateral mediastinal and hilar lymph node dissection, in accordance with the ESTS guidelines [22]. Surgery was performed through thoracotomy or thoracoscopy based on pre-operative imaging and surgeon preferences and skills.
Follow-up was assessed by outpatient visits including medical history, physical examination and enhanced contrast wb-CT scan every six months. The data were analysed to assess the impact of LNR after surgery on OS and DFS. LNR was defined as the ratio between the number of metastatic lymph nodes and the total number of lymph nodes retrieved; we chose our median LNR (0.29 or 29%) as the threshold for grouping, also considering previous studies [18-20].
Statistical Analysis
Statistical analysis was performed using SPSS 24.0 software (IBM SPSS Statistics for Macintosh, Version 24.0. Armonk, NY USA). Continuous variables are expressed as median and range, mean values ± SD; categorical variables were resumed with percentages. OS was calculated from the beginning of treatments to death or date of the last follow-up (31st March 2020); DFS was calculated for those patients who received resection from the starting treatment date to the date of the first evidence of recurrence. Survival probabilities were estimated using the Kaplan-Meier method and differences were compared with log-rank test. Cox proportional hazard regression was used to test the association with OS and DFS and covariates. Variables with p<0.20 at univariate analysis were further evaluated by multivariable analysis. Statistical significance for all tests was set a probability value of less than 0.05.
Results
I Clinical and Peri-Operative Findings
During the study period, 85 consecutive patients with cIIIA-B (N2) NSCLC underwent lung resection with systematic lymph node dissection after three to four weeks from the completion of NAC. Table 1 shows demographic, clinical and operative characteristics of the entire cohort. 63 (74.1%) patients had stage IIIA and 22 (25.9%) had stage IIIB comprising only patients with clinical T3N2 and T4N2. N2 disease was confirmed histologically by EBUS/EUS in 70 patients (82.4%) and by videomediastinoscopy in n=15 (17.6%).
Table 1: Demographic,
pre-operative and surgical characteristics of the study population.
|
Variable |
N=85 (%) mean±SD |
|
Males |
52 (61.2%) |
|
Age |
65.5±9.35 |
|
ECOG PS 0 1 2 |
49 (57.6%) 31 (36.5%) 5 (5.9%) |
|
Modified CCI 0 1 2 3 4 |
25 (29.4%) 30 (35.3%) 20 (23.5%) 9 (10.6%) 1 (1.2%) |
|
Comorbidities Hypertension CAD COPD Other solid tumor Diabetes Mellitus Vascular disease |
29 (34.1%) 7 (8.2%) 27 (31.7%) 5 (5.8%) 12 (14.1%) 3 (3.5%) |
|
Clinical stage IIIA IIIB |
63 (74.1%) 22 (25.9%) |
|
Pre-operative FEV1% |
85.4±18.2 |
|
Pre-operative DLCO% |
68±15.1 |
|
Invasive mediastinal staging EBUS/EUS VM |
70 (82.4%) 15 (17.6%) |
|
CDDP other |
48 (55.8%) 38 (44.2%) |
|
ADC SCC other |
48 (56.5%) 30 (35.3%) 7 (8.2%) |
|
RECIST response CR PR SD |
6 (7.1%) 51 (60%) 28 (32.9%) |
|
Surgical procedure Lobectomy/Bilobectomy Pneumonectomy Sublobar resection Sleeve lobectomy |
53 (62.3%) 14 (16.5%) 4 (4.7%) 14 (16.5%) |
|
Site of tumor Upper/Middle Lobe Lower lobe |
56 (65.9%) 29 (34.1%) |
|
Single station involvement More than one station involved |
42 (49.4%) 43 (50.6%) |
SD: Standard Deviation; ECOG PS: Eastern Cooperative Oncology Group
Performance Status; CCI: Charlson Comorbidity Index; CAD: Coronary Artery
Disease; COPD: Chronic Obstructive Pulmonary Disease; FEV1%: Forced Expiratory
Volume in 1 Second, DLCO%: Diffusing Capacity of the Lung for Carbon Monoxide,
Hb: Haemoglobin; EBUS/EUS: Endobronchial or Esophageal Ultrasound; VM:
Videomediastinoscopy; CDDP: Cisplatin Based Chemotherapy; ADC: Adenocarcinoma;
SCC: Squamous Cell Carcinoma; RECIST: Response Evaluation Criteria in Solid
Tumors; CR: Complete Response; PR: Partial Response; SD: Stable Disease.
We identified 42 patients (49.4%) with single station mediastinal involvement and 43 patients (50.6%) with involvement of more than one lymph nodal station. After chemotherapy, 6 patients (7.1%) had a complete clinical RECIST (response evaluation criteria in solid tumors) response whereas 60% obtained a partial response [23]. The remaining 32.9% patients showed a stable disease. Pneumonectomy was performed in 14 patients (16.5%) whereas sleeve-lobectomy and lobectomy represented respectively the 16.5% and 62.3%. Thoracoscopic lung resection (all lobectomies) was performed in n=16 (18.8%) patients. Complete resection (R0) was achieved in n=70 (82.3%) patients, while resection was microscopic incomplete in n=15 (17.7%). Table 2 shows the post-operative, pathological and oncological outcomes. Post-operative 30-day and 90-day mortality were 2.3% and 3.5% respectively; complications developed in 35 patients (41.2%), often minor and not-life-threatening (Clavien-Dindo class II n=23, 27%) [24].
Table 2: Post-operative, pathological and mid-long term oncological outcomes.
|
Variable |
N=85 (%) mean±SD |
Median (range) |
|
30 day/in hospital
mortality |
2 (2.3%) |
|
|
90-day/in-hospital
mortality |
3 (3.5%) |
|
|
Post-operative
complications |
35 (41.2%) |
|
|
Clavien-Dindo classification 2 3a 3b 4a 5 |
23 (27%) 4 (4.7%) 3 (3.5%) 2 (2.4%) 3 (3.5%) |
|
|
Hospital stay |
11.28±19.7 |
8 (3-180) |
|
ICU stay |
3.8±19.7 |
1 (1-160) |
|
Pathological tumor
diameter (mm) |
34±20.8 |
|
|
pT 0 1a 1b 1c 2a 2b 3 4 |
11 (12.9%) 7 (8.2%) 11 (12.9%) 9 (10.6%) 17 (20%) 11 (12.9%) 11 (12.9%) 8 (9.4%) |
|
|
pN 0 1 2 |
44 (51.8%) 14 (16.4%) 27 (31.8%) |
|
|
pN subclassification N1a N1b N2a1 N2a2 N2b |
11 (12.9%) 3 (3.5%) 16 (18.8%) 3 (3.5%) 8 (9.4%) |
|
|
Mediastinal involvement Single Two station Multiple station |
13 (48.1%) 9 (33.3%) 5 (18.6%) |
|
|
pStage 0 IA IB IIA IIB IIIA IIIB |
7 (8.2%) 14 (16.5%) 5 (5.9%) 5 (5.9%) 19 (22.4%) 30 (35.3%) 5 (5.9%) |
|
SD: Standard Deviation; ICU: Intensive Care Unit.
II Pathological Results and Nodal Assessment
Histological findings showed adenocarcinoma in 56.5% of patients and squamous cell carcinoma in 35.3%. A mean of 135.8 lymph nodes were dissected with a mean number of involved lymph node of 1.743.12. The median dissected lymph node stations were 6 (5-8). The final pathology analysis showed a complete pathological response (ypT0N0) in n=7 (8.2%) patients; 27 (31.8%) patients had persistent N2 disease (ypN2), whereas 58 (68.2%) patients had nodal downstaging to ypN1 (n=14, 16.4%) and to ypN0 (n=44, 51.8%), respectively. Single mediastinal station involvement was observed in 13 (48.1%) patients, while multiple level stations were involved in n=14 (51.9%). Among the 41 patients with ypN+, 28 (68.3%) had an LNR less than 0.29, while it was superior to 0.29 in 13 (15.3%). Regarding patients with LNR<0.29, 14 (15.3%) had ypN2 disease and 14 (16.5%) had pN1 disease, while all patients with LNR >0.29 had ypN2 disease (n=13). Adjuvant radiotherapy was performed in 4 patients (4.7%), 30 patients (35.3%) received adjuvant chemotherapy and 9 patients (10.6%) radio and chemotherapy.
III Survival Analysis
At the median follow up of 21 months (range 6 69 months), 30 patients (35.2%) had died (8 from tumor unrelated causes); 39 patients (45.8%) were alive and free of disease and 16 (18.8%) remained alive with recurrence. In particular, 8 (9.4%), 21 (24.7%) and 9 (10.6%) patients developed local, distant and combined recurrence, respectively.
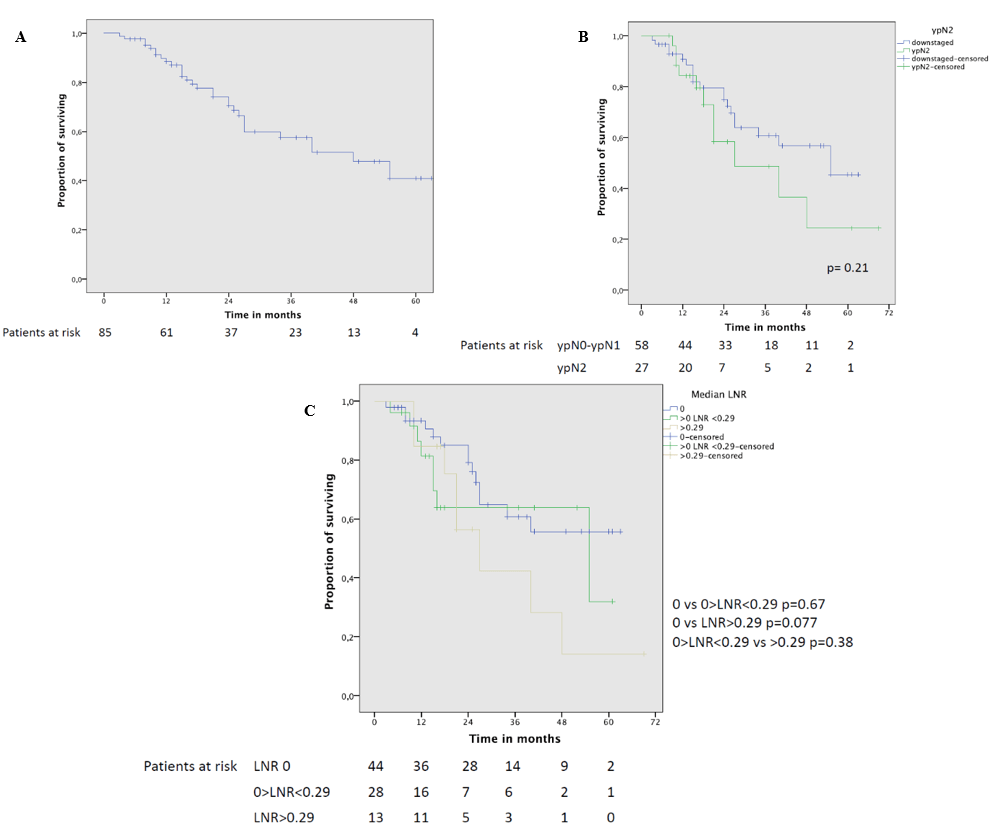
Figure 1: A) Overall survival curve of the whole cohort; B) overall survival curve of patients who had mediastinal downstaging and ypN2 disease; C) overall survival curve in patients with different LNR values.
The 5-year OS of the entire cohort was 41% with a median survival of 48 months (Figure 1A). Although OS was not statistically different between downstaged and ypN2 patients at the univariate analysis (Figure 1B), a worse 5-year OS was noted for patients with residual N2 disease (5-year OS 24.5% vs 45.5%, p=0.21, respectively). Patients with LNR 0 showed a trend toward better survival (Figure 1C) in comparison to patients with LNR>0.29 (5-year OS 56% VS 14%, p=0.077); this trend of significance was also confirmed at the multivariable analysis (HR 2.28; p=0.066).
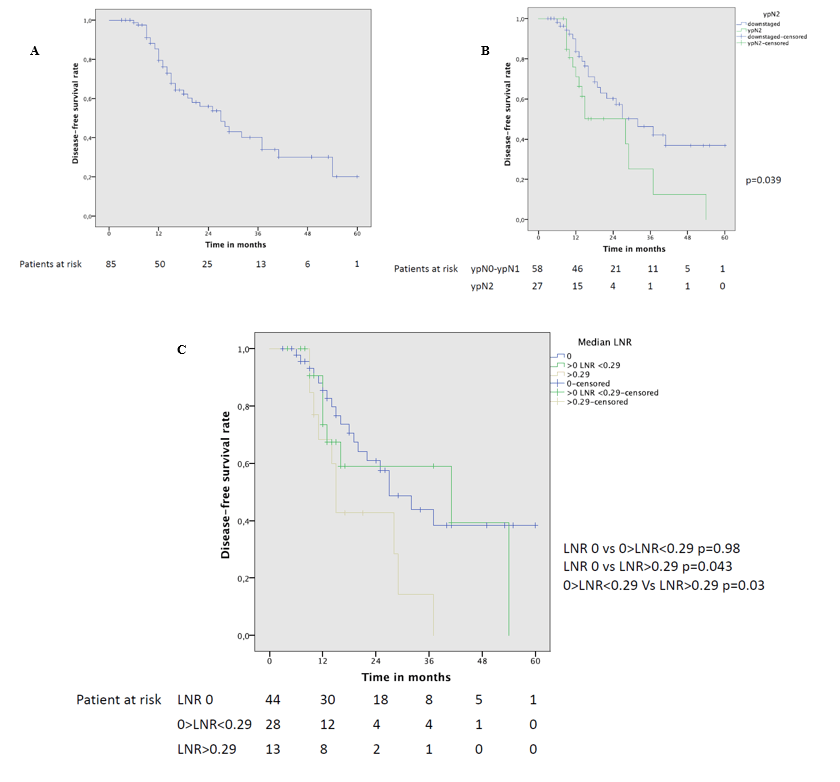
The 5-year DFS was 20% with a median DFS of 27 months (Figure 2A). At the univariate analysis (Table 3) a significantly worse DFS was observed for ypN2 patients (Figure 2B) compared to patients with lymph node downstaging (46% vs 25% 3-year DFS, p=0.039). Completeness of resection was associated with a significantly better 3-year DFS (42.5% vs 37.5%, p=0.025). Moreover, DFS was significantly different according to LNR: 3-year DFS was only 14% in patients with LNR>0.29 while it reached 44% in patients with LNR 0 and 59% in patients with LNR<0.29 (Figure 2C). LNR>0.29 remained the only significant predictor (HR 2.89; p=0.047) of reduced DFS at the multivariable analysis (Table 4).
Figure 2: A) Disease-free survival curve of the whole cohort; B) overall survival curve of patients who had mediastinal downstaging and ypN2 disease; C) disease-free survival curve in patients with different LNR values.
Table 3: Univariate analysis on disease-free survival.
|
Variable |
n (%) |
Median DFS in months |
3y-DFS |
5y-DFS |
p |
|
Whole cohort |
85 (100) |
27 |
40% |
20% |
|
|
Gender M F |
52 (61.2) 33 (38.8) |
37 19 |
53% 21% |
36% NA |
0.057 |
|
SCC Non-squamous |
30 (35.3) 55 (64.7) |
37 25 |
55% 30% |
38% NA |
0.25 |
|
Lobectomy/Sublobar Pneumonectomy |
67 (78.8) 14 (16.5) |
29 25 |
46% 29% |
22% NA |
0.66 |
|
Clinical stage IIIA IIIB |
63 (74.1) 22 (25.9) |
28 16 |
42.5% 33.5% |
NA 22% |
0.25 |
|
RECIST response CR+PR SD |
57 (67) 28 (33) |
27 29 |
44% 34% |
39% NA |
0.27 |
|
Downstaged ypN2 |
58 (68.2) 27 (37.8) |
32 28 |
46% 25% |
37% NA |
0.039 |
|
LNR0 LNR<0.29 LNR>0.29 |
44 (51.8) 28 (32.9) 13 (15.3) |
27 41 15 |
44% 59% 14% |
38% NA NA |
0vs<0.29 0.98 0vs>0.29 0.043 <0.29vs>0.29 0.03 |
|
ypN+ <0.29 ypN+ >0.29 |
28 (32.9) 13 (15.3) |
41 15 |
62% 14% |
NA NA |
0.03 |
|
N0+N1+N2 LNR <0.29 LNR >0.29 |
72 (84.7) 13 (15.3) |
27 15 |
46% 14% |
25% NA |
0.022 |
|
ypN1<0.29 ypN2<0.29 |
14 (16.5) 14 (16.5) |
41 54 |
64% 63% |
NA NA |
0.82
|
|
ypN0+N1<0.29 ypN2<0.29 |
58 (68.2) 14 (16.4) |
32 54 |
46% 63% |
37% NA |
0.48 |
|
Resection status R0 R1 |
70 (82.3%) 15 (17.7%) |
28 15 |
42.5% 37.5% |
23% NA |
0.025 |
|
PORT No treatment Yes |
43 (50.6) 4 (4.7) |
54 18 |
54% 25% |
36% NA |
0.15 |
|
Adjuvant chemotherapy No treatment Yes |
43 (50.6) 29 (34.1) |
54 28 |
54% 34% |
36% NA |
0.16 |
|
Adjuvant CHT+RT No treatment Yes |
43 (50.6) 9 (10.5) |
54 25 |
54% 37% |
36% NA |
0.18 |
|
Multistation ypN2 No yes |
13 (15.3) 14 (16.5) |
15 15 |
24.5% 24% |
NA NA |
0.45 |
DFS: Disease-Free Survival; NA: Not Available; SCC: Squamous Cell
Carcinoma; RECIST: Response Evaluation Criteria in Solid Tumors; CR: Complete
Response; PR: Partial Response; SD: Stable Disease; LNR: Lymph Node Ratio;
PORT: Post-Operative Radiotherapy; CHT: Chemotherapy; RT: Radiation Therapy.
Table 4: Univariate and multivariable Cox regression analysis about risk factors
of reduced disease-free survival.
|
|
Univariate analysis on DFS |
Multivariable analysis on DFS |
||||
|
Variable |
HR |
CI95% |
p |
HR |
CI95% |
p |
|
Sex female |
1.83 |
0.96-3.49 |
0.06 |
1.56 |
0.78-3.11 |
0.2 |
|
Age>70 years |
1.15 |
0.6-2.2 |
0.67 |
|
|
|
|
ADC |
1.56 |
0.76-3.2 |
0.22 |
|
|
|
|
pN0 pN1 pN2 |
ref 0.73 1.85 |
0.24-2.16 0.93-3.67 |
0.57 0.079 |
0.71 1.61 |
0.24-2.14 0.78-3.31 |
0.55 0.19 |
|
Pathological stage III |
2.03 |
1.07-3.88 |
0.03 |
1.76 |
0.89-3.47 |
0.1 |
|
LNR LNR 0 LNR <0.29 LNR >0.29 |
ref 1.2 2.4 |
0.54-2.67 1.1-5.23 |
0.65 0.027 |
1.14 2.89 |
0.51-2.56 1.23-6.77 |
0.73 0.047 |
|
ypN2 |
1.96 |
1.01-3.81 |
0.046 |
1.72 |
0.86-3.45 |
0.12 |
|
Adjuvant CHT |
1.5 |
0.78-2.91 |
0.22 |
|
|
|
|
PORT |
1.72 |
0.6-4.88 |
0.3 |
|
|
|
|
Adjuvant CHT-RT |
0.96 |
0.38-2.4 |
0.94 |
|
|
|
|
R1 |
2.32 |
1.07-5.04 |
0.032 |
1.58 |
0.5-4.98 |
0.43 |
DFS: Disease-Free Survival,
HR: Hazard Ratio; CI95%: Confidence Interval 95%; ADC: Adenocarcinoma; LNR:
Lymph Node Ratio; CHT: Chemotherapy; PORT: Post-Operative Radiation Therapy;
CHT-RT: Chemo-Radiation Therapy; R1: Microscopic Incomplete Resection.
When merging patients with ypN0-N1 and ypN2 with LNR <0.29 and comparing them with the subgroup of patients with ypN2 and LNR>0.29, a significant difference in 3-year DFS is found (46% vs 14%; p=0.022). No difference in 3-year DFS is found between patients with ypN1 and patients ypN2 with LNR<0.29 (64% vs 63%; p=0.82) or between patients with ypN0-N1 and patients with persistent N2 disease and LNR<0.29 group (p=0.48).
Discussion
The best multimodality treatment for resectable stage IIIA-B is still controversial, particularly concerning the role of surgery. This retrospective study reported the long-term outcomes of patients affected by stage IIIA-B (N2) NSCLC with pathologically proven mediastinal lymph node involvement who underwent a multimodality treatment including surgery after NAC. Our data suggest that this approach is well tolerated with an acceptable 90-day post-operative mortality, a low incidence of major complications and a good compliance with adjuvant treatments. Within this multimodal pathway we obtained a 41% 5-year OS and 20% 5-year DFS for the entire cohort. These results, in line with those reported in other previous publications showing a 5-year OS rates between 17% and 40% suggest that surgery is an appropriate option for selected patients when integrated in a multimodality approach [4, 25-29].
Due to the retrospective nature of this study, it is likely that a selection bias can have contributed to these favourable results, as patients with progressive disease during NAC were excluded from surgery. It should also be considered that all patients had a resectable disease at the start of NAC, which defines a better scenario compared to the baseline unresectable mediastinal lymph node involvement, as clearly shown by the results of the EORTC trial 08941 [3]. Previous studies showed that lymph nodal downstaging is one of the most powerful prognostic factors. The prognosis of patients with persistent N2 disease is constantly poorer in comparison with patients who achieved a mediastinal downstaging (5-year OS less than 20% vs 30-45%, respectively) [25-28]. In our series, we found only a trend toward better survival in patients with ypN0-N1 with 45% versus 24.5% 5-year OS in patients with ypN2. We found that the burden of nodal disease is a more significant determinant of survival compared to the nodal downstaging.
These data emerge from the analysis of the DFS: patients with LRN>0.29 had a significantly worse DFS compared to patients with lower LNR (3-year 14% vs 46%, p=0.022) and the multivariable analysis confirmed LNR>0.29 as the only independent prognostic factor for worse DFS. LNR<0.29 also showed a strong trend toward better OS at the multivariable analysis whereas the mediastinal downstaging did not have an influence on survival. This means that the subgroup of 27 patients with persistent N2 disease forms a heterogeneous group in which patients with less burden of nodal disease achieve a significantly better survival compared to patients with more burden of nodal disease and it could explain the controversial results reported in the literature with 0% 5-year OS in some series compared to around 20% 5-year OS in others [4, 20, 29-31].
In our study, when merging patients with ypN0, ypN1 and ypN2 with LNR <0.29 and comparing them with the subgroup of patients with ypN2 and LNR>0.29, we found a significant difference in 3-year DFS (46% vs 14%; p=0.022). No difference in 3-year DFS is found between patients with ypN1 and ypN2<0.29 (64% vs 63%, p=0.82) or between the ypN0-N1 and ypN2<0.29 group (46% vs 63%, p=0.48). These results suggest that not all patients with persistent N2 disease should be excluded from surgery, as some of these patients (those with low LNR) have a survival rate similar to patients with N1 disease and can be cured; moreover, patients with high burden of nodal disease should be absolutely selected for some adjuvant treatments. In our study population, the 60% of ypN2 patients and the 60% of patients with LNR>0.29 developed distant metastasis (data not shown) and then aggressive consolidative therapies (adjuvant chemotherapy and/or radiotherapy) should be administered to these patients [9, 17, 32].
The LNR emerged from our results as the most effective and reliable prognostic factors for OS and DFS, but how could the clinicians evaluate this finding pre-operatively? The use of invasive methods to restage the mediastinum like videomediastinoscopy have shown poor results in terms of accuracy, if already done before treatment [33]. Although EBUS/EUS could be considered an accurate method for restaging with a diagnostic accuracy of 77%, its use can only define the persistence of N2 disease, which is not a contraindication to surgery since some patients with persistent N2 disease and low burden of nodal involvement that can still benefit of it [34]. In fact, to evaluate the LNR is necessary to achieve a systematic lymph node dissection including the N1 stations, possible only with surgery. Some experiences showed a significative correlation between the decrease of PET-CT-scan avidity of the primary tumor and mediastinal lymph node downstaging [25, 35]. Hence, we believe that the integration of the clinical re-assessment with wb-CT-scan, PET-CT-scan and other clinical variables, are adequate to exclude the progression of disease and play a basilar role to indicate surgery [25, 35]. Moreover, the NCCN-guidelines suggest to proceed with surgery in case of persistent resectability and absence of tumor progression [1].
The traditional N classification considers only the lymphatic region involved and not the “burden of nodal disease”. The subclassification proposed by Park is a good compromise between the localization and the burden of nodal disease [13]. Some authors suggest the use of the number of the involved lymph node (as also used in other malignancy as breast, colonic or oesophageal cancer) while other consider the number of retrieved lymph nodes a critical factor to estimate the prognosis [15-19]. The LNR combines both concept into one evaluable parameter and its prognostic value has been shown in oncological settings different from NSCLC [15-18]. However, its influence after multimodality treatment has not been widely evaluated [16, 19, 20]. Our study clearly showed the prognostic role of high LNR value. Anyway, the use of LNR needs some consideration: first of all, a systematic lymph node dissection is the natural prerequisite for a reliable LNR; second, different thresholds have been reported in the literature ranging from 15-50% [1, 15-20, 22]. Larger studies are needed to clarify which is the best cut-off value for LNR to predict prognosis and to indicate adjuvant treatments.
Our study has some limitations: first of all, it is a retrospective study with its inherent potential selection bias. Second, LNR is affected by the type of lymph node dissection and then could differ between surgeons and institutions even if international guidelines suggest the use of systematic lymph node dissection for NSCLC; third, this is a cohort study without control group and then differences in baseline patient characteristics of our cohort should be considered when comparing our results with those of other published series; moreover, we did not perform a comparison of the outcomes between NAC followed by surgery and chemo-radiotherapy followed by immunotherapy [1, 22, 36]. On the other hand, the short study period (2014-2020) can be considered an advantage for the homogeneity of the diagnostic path used with modern techniques of staging and restaging (all with wb-CT-scan and mostly with PET-CT-scan), the adoption of modern surgical and anaesthetic techniques and also for the latest therapeutic protocols in both neoadjuvant and adjuvant settings.
Conclusion
Surgery after NAC in patients with Stage IIIA-B (N2) NSCLC can be nowadays performed with an acceptable morbidity and mortality. Patients with persistent N2 disease after NAC and surgery can have acceptable outcomes and this finding is particularly true for patients with low burden of nodal disease that can be defined by the LNR. High value of LNR had a strong negative influence on survival and represents a clear indication to adjuvant treatment. Some well-designed or prospective large-scale studies are necessary to verify the prognostic role of LNR and to assess the right cut-off of this potentially useful tool to address patients to a tailored adjuvant treatment.
Funding
None.
Conflicts of Interest
None.
Author Contributions
Stefano Bongiolatti: Conceptualization, Methodology, Data curation, Formal analysis, Software, Validation, Writing-original draft; Francesca Mazzoni: Data curation, Investigation, Validation; Alessandro Gonfiotti: Methodology, Writing-review & editing, Validation; Alberto Salvicchi: Data curation, Investigation,Validation; Katia Ferrari: Data curation, Investigation, Validation; Vieri Scotti: Data curation, Investigation,Validation; Luca Voltolini: Conceptualization, Writing-review & editing, Validation.
Abbreviations and Acronyms
NSCLC: Non-Small-Cell Lung Cancer
NAC: Neoadjuvant Chemotherapy
MBT: Multidisciplinary Tumor Board
ypN2: persistent N2 disease
LNR: Lymph Node Ratio
OS: Overall Survival
DFS: Disease-Free Survival
EBUS/EUS: Endobronchial Ultrasound/Esophageal Ultrasound
Article Info
Article Type
Research ArticlePublication history
Received: Mon 05, Apr 2021Accepted: Mon 26, Apr 2021
Published: Mon 31, May 2021
Copyright
© 2023 Alberto Salvicchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2021.05.03
Author Info
Stefano Bongiolatti Francesca Mazzoni Alessandro Gonfiotti Alberto Salvicchi Domenico Viggiano Katia Ferrari Vieri Scotti Luca Voltolini
Corresponding Author
Alberto SalvicchiThoracic Surgery Unit, Careggi University Hospital, Florence, Italy
Figures & Tables
Table 1: Demographic,
pre-operative and surgical characteristics of the study population.
|
Variable |
N=85 (%) mean±SD |
|
Males |
52 (61.2%) |
|
Age |
65.5±9.35 |
|
ECOG PS 0 1 2 |
49 (57.6%) 31 (36.5%) 5 (5.9%) |
|
Modified CCI 0 1 2 3 4 |
25 (29.4%) 30 (35.3%) 20 (23.5%) 9 (10.6%) 1 (1.2%) |
|
Comorbidities Hypertension CAD COPD Other solid tumor Diabetes Mellitus Vascular disease |
29 (34.1%) 7 (8.2%) 27 (31.7%) 5 (5.8%) 12 (14.1%) 3 (3.5%) |
|
Clinical stage IIIA IIIB |
63 (74.1%) 22 (25.9%) |
|
Pre-operative FEV1% |
85.4±18.2 |
|
Pre-operative DLCO% |
68±15.1 |
|
Invasive mediastinal staging EBUS/EUS VM |
70 (82.4%) 15 (17.6%) |
|
CDDP other |
48 (55.8%) 38 (44.2%) |
|
ADC SCC other |
48 (56.5%) 30 (35.3%) 7 (8.2%) |
|
RECIST response CR PR SD |
6 (7.1%) 51 (60%) 28 (32.9%) |
|
Surgical procedure Lobectomy/Bilobectomy Pneumonectomy Sublobar resection Sleeve lobectomy |
53 (62.3%) 14 (16.5%) 4 (4.7%) 14 (16.5%) |
|
Site of tumor Upper/Middle Lobe Lower lobe |
56 (65.9%) 29 (34.1%) |
|
Single station involvement More than one station involved |
42 (49.4%) 43 (50.6%) |
SD: Standard Deviation; ECOG PS: Eastern Cooperative Oncology Group
Performance Status; CCI: Charlson Comorbidity Index; CAD: Coronary Artery
Disease; COPD: Chronic Obstructive Pulmonary Disease; FEV1%: Forced Expiratory
Volume in 1 Second, DLCO%: Diffusing Capacity of the Lung for Carbon Monoxide,
Hb: Haemoglobin; EBUS/EUS: Endobronchial or Esophageal Ultrasound; VM:
Videomediastinoscopy; CDDP: Cisplatin Based Chemotherapy; ADC: Adenocarcinoma;
SCC: Squamous Cell Carcinoma; RECIST: Response Evaluation Criteria in Solid
Tumors; CR: Complete Response; PR: Partial Response; SD: Stable Disease.
Table 2: Post-operative, pathological and mid-long term oncological outcomes.
|
Variable |
N=85 (%) mean±SD |
Median (range) |
|
30 day/in hospital
mortality |
2 (2.3%) |
|
|
90-day/in-hospital
mortality |
3 (3.5%) |
|
|
Post-operative
complications |
35 (41.2%) |
|
|
Clavien-Dindo classification 2 3a 3b 4a 5 |
23 (27%) 4 (4.7%) 3 (3.5%) 2 (2.4%) 3 (3.5%) |
|
|
Hospital stay |
11.28±19.7 |
8 (3-180) |
|
ICU stay |
3.8±19.7 |
1 (1-160) |
|
Pathological tumor
diameter (mm) |
34±20.8 |
|
|
pT 0 1a 1b 1c 2a 2b 3 4 |
11 (12.9%) 7 (8.2%) 11 (12.9%) 9 (10.6%) 17 (20%) 11 (12.9%) 11 (12.9%) 8 (9.4%) |
|
|
pN 0 1 2 |
44 (51.8%) 14 (16.4%) 27 (31.8%) |
|
|
pN subclassification N1a N1b N2a1 N2a2 N2b |
11 (12.9%) 3 (3.5%) 16 (18.8%) 3 (3.5%) 8 (9.4%) |
|
|
Mediastinal involvement Single Two station Multiple station |
13 (48.1%) 9 (33.3%) 5 (18.6%) |
|
|
pStage 0 IA IB IIA IIB IIIA IIIB |
7 (8.2%) 14 (16.5%) 5 (5.9%) 5 (5.9%) 19 (22.4%) 30 (35.3%) 5 (5.9%) |
|
SD: Standard Deviation; ICU: Intensive Care Unit.
Table 3: Univariate analysis on disease-free survival.
|
Variable |
n (%) |
Median DFS in months |
3y-DFS |
5y-DFS |
p |
|
Whole cohort |
85 (100) |
27 |
40% |
20% |
|
|
Gender M F |
52 (61.2) 33 (38.8) |
37 19 |
53% 21% |
36% NA |
0.057 |
|
SCC Non-squamous |
30 (35.3) 55 (64.7) |
37 25 |
55% 30% |
38% NA |
0.25 |
|
Lobectomy/Sublobar Pneumonectomy |
67 (78.8) 14 (16.5) |
29 25 |
46% 29% |
22% NA |
0.66 |
|
Clinical stage IIIA IIIB |
63 (74.1) 22 (25.9) |
28 16 |
42.5% 33.5% |
NA 22% |
0.25 |
|
RECIST response CR+PR SD |
57 (67) 28 (33) |
27 29 |
44% 34% |
39% NA |
0.27 |
|
Downstaged ypN2 |
58 (68.2) 27 (37.8) |
32 28 |
46% 25% |
37% NA |
0.039 |
|
LNR0 LNR<0.29 LNR>0.29 |
44 (51.8) 28 (32.9) 13 (15.3) |
27 41 15 |
44% 59% 14% |
38% NA NA |
0vs<0.29 0.98 0vs>0.29 0.043 <0.29vs>0.29 0.03 |
|
ypN+ <0.29 ypN+ >0.29 |
28 (32.9) 13 (15.3) |
41 15 |
62% 14% |
NA NA |
0.03 |
|
N0+N1+N2 LNR <0.29 LNR >0.29 |
72 (84.7) 13 (15.3) |
27 15 |
46% 14% |
25% NA |
0.022 |
|
ypN1<0.29 ypN2<0.29 |
14 (16.5) 14 (16.5) |
41 54 |
64% 63% |
NA NA |
0.82
|
|
ypN0+N1<0.29 ypN2<0.29 |
58 (68.2) 14 (16.4) |
32 54 |
46% 63% |
37% NA |
0.48 |
|
Resection status R0 R1 |
70 (82.3%) 15 (17.7%) |
28 15 |
42.5% 37.5% |
23% NA |
0.025 |
|
PORT No treatment Yes |
43 (50.6) 4 (4.7) |
54 18 |
54% 25% |
36% NA |
0.15 |
|
Adjuvant chemotherapy No treatment Yes |
43 (50.6) 29 (34.1) |
54 28 |
54% 34% |
36% NA |
0.16 |
|
Adjuvant CHT+RT No treatment Yes |
43 (50.6) 9 (10.5) |
54 25 |
54% 37% |
36% NA |
0.18 |
|
Multistation ypN2 No yes |
13 (15.3) 14 (16.5) |
15 15 |
24.5% 24% |
NA NA |
0.45 |
DFS: Disease-Free Survival; NA: Not Available; SCC: Squamous Cell
Carcinoma; RECIST: Response Evaluation Criteria in Solid Tumors; CR: Complete
Response; PR: Partial Response; SD: Stable Disease; LNR: Lymph Node Ratio;
PORT: Post-Operative Radiotherapy; CHT: Chemotherapy; RT: Radiation Therapy.
Table 4: Univariate and multivariable Cox regression analysis about risk factors
of reduced disease-free survival.
|
|
Univariate analysis on DFS |
Multivariable analysis on DFS |
||||
|
Variable |
HR |
CI95% |
p |
HR |
CI95% |
p |
|
Sex female |
1.83 |
0.96-3.49 |
0.06 |
1.56 |
0.78-3.11 |
0.2 |
|
Age>70 years |
1.15 |
0.6-2.2 |
0.67 |
|
|
|
|
ADC |
1.56 |
0.76-3.2 |
0.22 |
|
|
|
|
pN0 pN1 pN2 |
ref 0.73 1.85 |
0.24-2.16 0.93-3.67 |
0.57 0.079 |
0.71 1.61 |
0.24-2.14 0.78-3.31 |
0.55 0.19 |
|
Pathological stage III |
2.03 |
1.07-3.88 |
0.03 |
1.76 |
0.89-3.47 |
0.1 |
|
LNR LNR 0 LNR <0.29 LNR >0.29 |
ref 1.2 2.4 |
0.54-2.67 1.1-5.23 |
0.65 0.027 |
1.14 2.89 |
0.51-2.56 1.23-6.77 |
0.73 0.047 |
|
ypN2 |
1.96 |
1.01-3.81 |
0.046 |
1.72 |
0.86-3.45 |
0.12 |
|
Adjuvant CHT |
1.5 |
0.78-2.91 |
0.22 |
|
|
|
|
PORT |
1.72 |
0.6-4.88 |
0.3 |
|
|
|
|
Adjuvant CHT-RT |
0.96 |
0.38-2.4 |
0.94 |
|
|
|
|
R1 |
2.32 |
1.07-5.04 |
0.032 |
1.58 |
0.5-4.98 |
0.43 |
DFS: Disease-Free Survival,
HR: Hazard Ratio; CI95%: Confidence Interval 95%; ADC: Adenocarcinoma; LNR:
Lymph Node Ratio; CHT: Chemotherapy; PORT: Post-Operative Radiation Therapy;
CHT-RT: Chemo-Radiation Therapy; R1: Microscopic Incomplete Resection.
References
1.
Ettinger DS,
Aisner DL, Wood DE, Akerley W, Bauma J et al. (2018) NCCN Guidelines Insights:
Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 16:
807-821. [Crossref]
2.
Albain KS, Swann
RS, Rusch VW, Turrisi AT, Shepherd FA et al. (2009) Radiotherapy plus
chemotherapy with or without surgical resection for stage III non-small-cell
lung cancer: a phase III randomised controlled trial. Lancet 374:
379-386. [Crossref]
3.
van Meerbeeck JP,
Kramer GWPM, van Schil PEY, Legrand C, Smit EF et al. (2007) Randomized
controlled trial of resection versus radiotherapy after induction chemotherapy
in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 99:
442-450. [Crossref]
4.
Eberhardt WE,
Pöttgen C, Gauler TC, Friedel G, Veit S et al. (2015) Phase III Study of
Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With
Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After
Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin
Oncol 33: 4194-4201. [Crossref]
5.
Voltolini L, Luzzi L, Ghiribelli C, Paladini P, Di
Bisceglie M et al. (2001) Results of
induction chemotherapy followed by surgical resection in patients with stage
IIIA (N2) non-small cell lung cancer: the importance of the nodal down-staging
after chemotherapy. Eur J Cardiothorac Surg 20: 1106-1112. [Crossref]
6.
Song WA, Zhou NK,
Wang W, Chu XY, Liang CY et al. (2010) Survival benefit of neoadjuvant
chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13
randomized control trials. J Thorac Oncol 5: 510-516. [Crossref]
7.
Früh M, Betticher
DC, Stupp R, Xyrafas A, Peters S et al. (2019) Swiss Group for Clinical Cancer
Research (SAKK). Multimodal Treatment in Operable Stage III NSCLC: A Pooled
Analysis on Long-Term Results of Three SAKK trials (SAKK 16/96, 16/00, and
16/01). J Thorac Oncol 14: 115-123. [Crossref]
8.
Betticher DC, Hsu
Schmitz SF, Tötsch M, Hansen E, Joss C et al. (2003) Mediastinal lymph node
clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of
survival in patients with stage IIIA pN2 non-small-cell lung cancer: a
multicenter phase II trial. J Clin Oncol 21: 1752-1759. [Crossref]
9.
Spicer JD,
Shewale JB, Nelson DB, Mitchell KG, Bott MJ et al. (2019) Multimodality Therapy
for N2 Non-Small Cell Lung Cancer: An Evolving Paradigm. Ann Thorac Surg
107: 277-284. [Crossref]
10. Steger V, Walker T, Mustafi M, Lehrach K, Kyriss T et
al. (2012) Surgery on unfavourable persistent N2/N3 non-small-cell lung cancer
after trimodal therapy: do the results justify the risk? Interact Cardiovasc
Thorac Surg 15: 948-953. [Crossref]
11. Port JL, Korst RJ, Lee PC, Levin MA, Becker DE et al.
(2005) Surgical resection for residual N2 disease after induction chemotherapy.
Ann Thorac Surg 79: 1686-1690. [Crossref]
12. De Leyn P, Vansteenkiste J, Deneffe G, Van Raemdonck
D, Coosemans W et al. (1999) Result of induction chemotherapy followed by
surgery in patients with stage IIIA N2 NSCLC: importance of pre-treatment
mediastinoscopy. Eur J Cardiothorac Surg 15: 608-614. [Crossref]
13. Park BK, Kim TH, Shin S, Kim HK, Choi YS et al. (2019)
Recommended Change in the N Descriptor Proposed by the International
Association for the Study of Lung Cancer: A Validation Study. Thorac Oncol
14: 1962-1969. [Crossref]
14. Matsuguma H, Oki I,
Nakahara R, Ohata N, Igarashi S et al. (2012)
Proposal of new nodal classifications for non-small-cell lung cancer based on
the number and ratio of metastatic lymph nodes. Eur J Cardiothorac Surg
41: 19-24. [Crossref]
15. Zhao Y, Li G, Zheng D,
Jia M, Dai W et al. (2017) The prognostic
value of lymph node ratio (lnr) and log odds of positive lymph nodes (lodds) in
patients with lung adenocarcinoma. J Thorac Cardiovasc Surg 153:
702-709.e1. [Crossref]
16. Taylor M, LaPar DJ, Thomas CJ, Persinger M, Stelow EB
et al. (2013) Lymph Node Ratio Predicts Recurrence and Survival After R0
Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 96: 1163-1170.
[Crossref]
17. Urban D, Bar J, Solomon B, Ball D (2013) Lymph node
ratio may predict the benefit of postoperative radiotherapy in non-small-cell
lung cancer. J Thorac Oncol 8: 940-946. [Crossref]
18. Chiappetta M, Leuzzi G,
Sperduti I, Bria E, Mucilli F et al. (2019)
Lymph-node ratio predicts survival among the different stages of non-small-cell
lung cancer: a multicentre analysis. Eur J Cardiothorac Surg 55:
405-412. [Crossref]
19. Shinde A, Horne ZD, Li R,
Glaser S, Massarelli E et al. (2019)
Optimal adjuvant therapy in clinically N2 non-small cell lung cancer patients
undergoing neoadjuvant chemotherapy and surgery: The importance of pathological
response and lymph node ratio. Lung Cancer 133: 136-143. [Crossref]
20. Haager B, Wiesemann S, Passlick B, Schmid S (2018)
Prognostic value of lymph node ratio after induction therapy in stage IIIA/N2
non-small cell lung cancer: a monocentric clinical study. J Thorac Dis
10: 3225-3231. [Crossref]
21. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW,
Vallières E et al. (2017) The IASLC Lung Cancer Staging Project: External
Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of
the TNM Classification of Lung Cancer. J Thorac Oncol 12: 1109-1121. [Crossref]
22. De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B
et al. (2014) Revised ESTS guidelines for preoperative mediastinal lymph node
staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 45:
787-798. [Crossref]
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH,
Sargent D et al. (2009) New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247. [Crossref]
24. Dindo D, Demartines N, Clavien PA (2004)
Classification of surgical complications: a new proposal with evaluation in a
cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213.
[Crossref]
25. Dooms C, Verbeken E, Stroobants S, Nackaerts K, De
Leyn P et al. (2008) Prognostic stratification of stage IIIA-N2 non-small-cell
lung cancer after induction chemotherapy: a model based on the combination of
morphometric-pathologic response in mediastinal nodes and primary tumor
response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. Clin
Oncol 26: 1128-1134. [Crossref]
26. Barlési F, Doddoli C,
Torre JP, Giudicelli R, Fuentes P et al. (2005)
Comparative prognostic features of stage IIIAN2 and IIIB non-small-cell lung
cancer patients treated with surgery after induction therapy. Eur J
Cardiothorac Surg 28: 629-634. [Crossref]
27. Bueno R, Richards WG, Swanson SJ, Jaklitsch MT,
Lukanich JM et al. (2000) Nodal stage after induction therapy for stage IIIA
lung cancer determines patient survival. Ann Thorac Surg 70: 1826-1831.
[Crossref]
28. Martin J, Ginsberg RJ, Venkatraman ES, Bains MS,
Downey RJ et al. (2002) Long-term results of combined-modality therapy in
resectable non-small-cell lung cancer. J Clin Oncol 20: 1989-1995. [Crossref]
29. Decaluwé H, De Leyn P, Vansteenkiste J, Dooms C, Van
Raemdonck D et al. (2009) Surgical multimodality treatment for baseline
resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal
lymph node involvement and impact on survival. Eur J Cardiothorac Surg
36: 433-439. [Crossref]
30. Renaud S, Falcoz PE, Olland A, Reeb J, Santelmo N et al. (2015) Mediastinal
downstaging after induction treatment is not a significant prognostic factor to
select patients who would benefit from surgery: the clinical value of the lymph
node ratio. Interact Cardiovasc Thorac Surg 20: 222-227. [Crossref]
31. Higgins KA, Chino JP, Ready N, Onaitis MW, Berry MF et
al. (2011) Persistent N2 disease after neoadjuvant chemotherapy for
non-small-cell lung cancer. J Thorac Cardiovasc Surg 142: 1175-1179. [Crossref]
32. Amini A, Correa AM, Komaki R, Chang JY, Tsao AS et al.
(2012) The role of consolidation therapy for stage III non-small cell lung
cancer with persistent N2 disease after induction chemotherapy. Ann Thorac
Surg 94: 914-920. [Crossref]
33. De Leyn P, Stroobants S, De Wever W, Lerut T,
Coosemans W et al. (2006) Prospective comparative study of integrated positron
emission tomography-computed tomography scan compared with remediastinoscopy in
the assessment of residual mediastinal lymph node disease after induction
chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung
cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 24: 3333-3339. [Crossref]
34. Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A
et al. (2008) Endobronchial ultrasound with transbronchial needle aspiration
for restaging the mediastinum in lung cancer. J Clin Oncol 26:
3346-3350. [Crossref]
35. Kamel MK, Rahouma M, Ghaly G, Nasar A, Port JL et al.
(2017) Clinical Predictors of Persistent Mediastinal Nodal Disease After
Induction Therapy for Stage IIIA N2 Non-Small Cell Lung Cancer. Ann Thorac
Surg 103: 281-286. [Crossref]
36. Antonia SJ, Villegas A,
Daniel D, Vicente D, Murakami S et al. (2017)
Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N
Engl J Med 377: 1919-1929. [Crossref]

