Hypotensive Effect Induced by Mandibular Extension in Aged, Hypertensive Humans and Rats
A B S T R A C T
Objectives: Previous research has shown that submaximal mouth opening by mandibular extension (ME) is followed by a prolonged reduction in blood pressure. This effect was observed in young and adult normotensive and hypertensive rats and in young normotensive human subjects.
Methods: We assessed the effects of a ME for 10 minutes obtained with a fixed mouth opener in both hypertensive adult humans (aged 55 years or older) and elderly (6-7 months) anaesthetized, hypertensive rats (SHR). Blood pressure and heart rate were measured every 10 minutes by non-invasive automatic recorders for 30 minutes before and 120 minutes after the procedure. Nine human hypertensive subjects (7 experimental and 2 controls) and seven spontaneously hypertensive rats (5 experimental and 2 controls) were tested.
Results: A statistically significant reduction in systolic blood pressure (SBP), mean arterial blood pressure (MABP) and heart rate (HR) was observed after ME in the seven hypertensive human subjects, in whom an average decrease of 15 mmHg for SBP, 10 mmHg for MABP and 7 bpm for HR, was observed. A similar hypotensive effect was recorded in spontaneously hypertensive rats that displayed a statistically significant decrease of SBP, DBP and MABP, amounting to about 40-50 mmHg.
Conclusion: This study provides the first evidence that ME has an important and prolonged hypotensive effect when applied to subjects with high blood pressure, making their arterial blood pressure decrease toward normal values for at least two hours.
Keywords
Blood pressure, heart rate, hypertension, stretching
Introduction
Several studies, starting from the pioneering work by Kumada and co-workers in rabbits have shown that manipulation of the facial region through electrical stimulation of the trigeminal system or maxillofacial surgery can elicit a reflex leading to a sudden onset of a number of physiological responses including bradycardia, hypotension, apnea and gastric hypermotility [1, 2]. Since the afferent branch is represented by the trigeminal nerve, these reflexes are also collectively referred to as “trigemino cardiac reflexes” [3-5].
Over the last years, our research group has built upon these observations to demonstrate that a reduction of arterial blood pressure and heart rate could also be obtained through a non-invasive technique consisting of a prolonged opening of the mouth, which we called mandibular extension (ME). Our studies have proved evidence for a marked hypotensive effect in anaesthetized young and adult rats, in which a decrease of about 20 mmHg in mean arterial blood pressure was observed and a less marked hypotensive effect in normotensive young human subjects [6-10]. Given these results, the next step was to explore whether ME could also have an effect in a hypertensive state. Recently, we have observed that ME is followed by a prolonged decline in mean blood pressure also in rats in which hypertension was induced by dexamethasone administration (20 µg/kg/day, subcutaneously for 7 days) and in spontaneously hypertensive rats (SHR). In both cases, the treatment induced a marked reduction in mean arterial blood pressure, which was much greater compared to that observed in normotensive rats [11].
In SHRs, blood pressure progressively and spontaneously increases starting from 4 up to 14 weeks of life so that systolic blood pressure rises to 180-200 mmHg, which persists for the entire life [12]. Also, the incidence of hypertension in humans is known to increase with age and essential hypertension is a condition typically associated with aging. In addition, the prevalence of hypertension and its impact on health are increasing over the last years because of the growing life expectancy [13]. In the present study, we aimed to extend our previous findings by applying ME to rats and human subjects which were in a hypertensive condition and of mature age (6-7 months for rats; > 55 years for human subjects).
Materials and Methods
I Experiments in Rats
Male SHRs (n = 7) were obtained from Charles River (Calco, SO, Italy). The animals were six- to seven-month-old and weighed 250-350 g. They were housed in polyethylene cages, under a 12/12 h light/dark cycle (light 8:00-20:00 h) at constant temperature (24 ± 1°C) and humidity (60 ± 5%) with free access to food and water. During the procedure, they were maintained anaesthetized with an intraperitoneal injection (10 mg/0.1 Kg b.w.) of pentothal sodium (MSD Italia, Aprilia, LT) for the entire experimental period, administering about 0.8 ml of the drug solution in each animal at the final concentration of 40 mg/ml. A single 10 minutes mandibular extension was induced in 5 rats using an appropriately U-shaped spring device placed between the superior and inferior dental arches of the animal as previously described [6-8]. Briefly, the spring device consisted of two thin layers covered with a silicone elastomer (Sylgard, Dow Corning, Midland, MI) coupled to an adjustable spring, allowing to open the month without muscle fatigue. SBP (systolic blood pressure), DBP (diastolic blood pressure), MABP (mean arterial BP) and HR (heart rate) were obtained by means of the non-invasive Mouse and Rat Tail Cuff Method Blood Pressure Systems (MRBP, IITC, Life Science Inc, Los Angeles; CA, USA) that allows an indirect measure of arterial blood pressure from the animal’s tail.
Therefore, the rats were introduced in a plexiglass tube that allowed to maintain the animal in a prone position with the tail inserted in a cuff containing the transducer under controlled temperature (about 28°C). To reduce the stress due to immobilization, the animals were trained to remain in the tube for five days prior to the experiment. For this, they were placed inside the plexiglass tube once a day for ten minutes while the cuff for measuring blood pressure was applied to their tail. On the day of the experiment, arterial blood pressure and heart rate were initially measured in conscious rats inserted in the tube. Immediately afterwards, the rat was anaesthetized and arterial blood pressure was then measured again 10 minutes after anaesthesia induction (B, basal values, in Figure 1). After the second blood pressure measurement, mandibular extension treatment was applied for 10 minutes (T, treatment, in Figure 1), after which arterial blood pressure was recorded every 20 minutes for 120 minutes (post-treatment period). Two rats (controls) were only anaesthetized and monitored for a period equivalent to the duration of the experiment. No animal died during the experimental procedures.
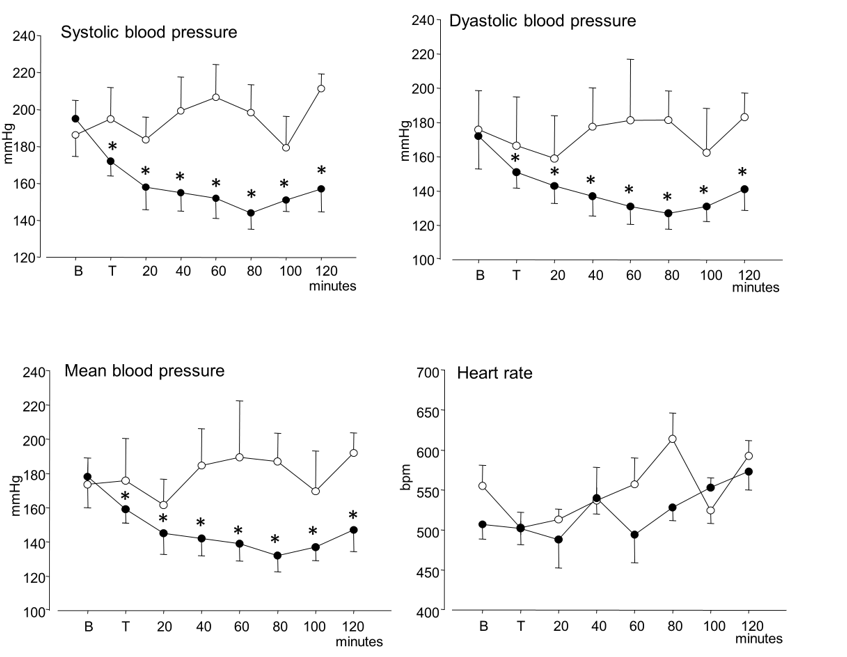
Figure 1: Time courses of systolic blood pressure, diastolic blood pressure, mean arterial blood pressure and heart rate (mean ± SEM) in anaesthetized hypertensive rats. Open circles: controls; black circles: after mandibular extension. “B” indicates the baseline value and “T” the value recorded at the end of the 10 minutes mandibular extension. Asterisks (*) indicate significant differences from baseline values in post-hoc comparisons following one-way ANOVA.
II Experiments in Humans
All the studies were performed between 9:00 and 13:00 at the Outpatient Clinic of the Fondazione G. Monasterio, in a room where the ambient temperature was controlled between 22°C and 24°C. Experimental subjects were selected among a larger group of patients that underwent a cardiologic visit at the Fondazione and were found to be free of major diseases (except for arterial hypertension), including dental and trigeminal problems and temporomandibular disorders. Most importantly, recruited patients must not be under pharmacological hypotensive treatments and have consumed caffeine, tobacco, tea and alcohol in the 2 hours before the study - two criteria that largely reduced the subjects available for the study. An automated blood pressure recorder (Spacelabs 9027) was applied to their non-dominant arm and blood pressure and heart rate were thereafter recorded every 10 minutes throughout the study. During the entire study, subjects were comfortably seated and remained alone watching some nature documentaries (BBC Worldwide), devoid of strong emotional contents, on a laptop screen.
A mandibular extension was induced in 7 subjects by means of a commercial mouth opener device, used in dental practice (Molt; Asa Dental; Bozzano Camaiore Lucca). The instrument was applied between the upper and lower incisor teeth and calibrated for a fixed opening at 60% of maximal mouth opening of the subject, as measured by the interincisal distance, and kept in place for 10 min. The remaining 2 subjects acted as controls and kept a sterile wooden tongue depressor between upper and lower incisor teeth. Each recording session lasted 170 minutes: 40 minutes before ME, followed by 10 minutes during ME and a subsequent period of 120 minutes after the test. Out of the four-blood pressure and heart rate measurements recorded before the test in each individual, the first one was discarded and the following three were averaged and used as the baseline reference values. ME device was applied immediately after the fourth blood pressure recording.
III Statistical Analysis
Data were tested for normal distribution with the Kolmogorov-Smirnov test. Due to the normality of distribution, statistical analysis was performed using one-way ANOVA with repeated measures to test differences over time. When the ANOVA revealed a statistically significant effect (P < 0.05), the Holm Sidak test was made for “post hoc” comparisons. All analyses were done with the statistical package Sigma Stat, version 3.5 (Jandel Corporation San Mateo, CA).
Table 1: Mean ± SEM values of arterial blood pressure.
|
N =6 |
SBP |
DBP |
MEAN |
HR |
|
Awake rats |
200±6.5 |
173±8.9 |
177±8.4 |
484±25.7 |
|
Anaesthetized rats |
197±7.0 |
177±8.8 |
182±7.5 |
532±13.5 |
Results
I Experiments in Rats
As shown in (Table 1), no significant differences were observed in the values of all parameters recorded in SHR between awake and anaesthetized conditions (in one rat, BP and HR were not recorded in awake condition). Figure 1 describes the time course of the mean values of SBP, DBP, MABP and HR in anaesthetized SHR and post-hoc comparisons that attained statistical significance are reported in the figure by asterisks. No significant change was present in control SHRs for the entire observation period of 140 minutes. Conversely, in ME-treated rats, a marked and significant (P<0.001) decrease was observed in SBP, DPB and MABP, which started immediately after treatment. SBP declined from 193±9.8 mmHg to 157±12.2 mmHg and attained a nadir at 144 ±8.8 mmHg. DBP declined from 172±9.2 mmHg to 141±12.3 mmHg and attained a nadir at 127 ±9.2 mmHg. MABP declined from 178±11.2 mmHg to 147±12.7 mmHg and attained a nadir at 132 ±9.4 mmHg. Heart rate increased no significantly over time, either for control or ME-treated rats (Figure 1).
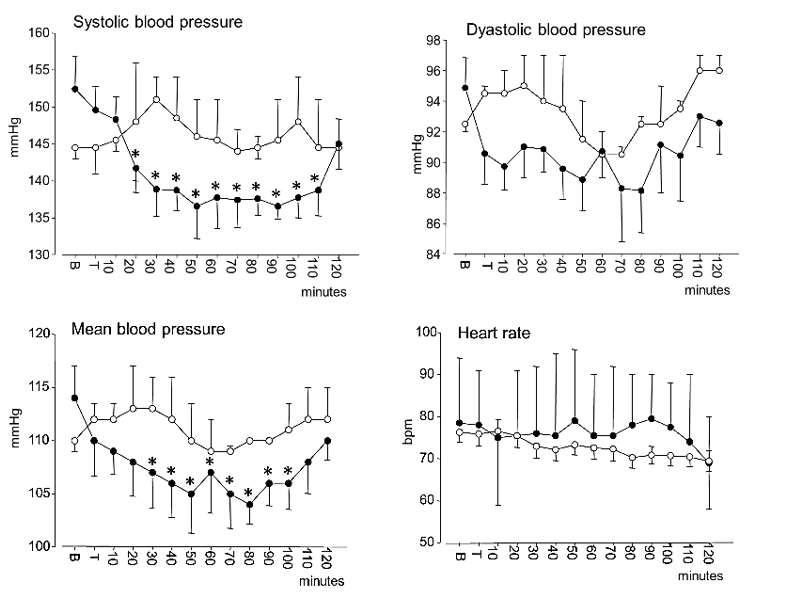
Figure 2: Time courses of systolic blood pressure, diastolic blood pressure, mean arterial blood pressure and heart rate (mean ± SEM) in hypertensive subjects. Open circles: controls; black circles: after mandibular extension. “B” indicates the baseline value and “T” the value recorded at the end of the 10 minutes mandibular extension. Asterisks (*) indicate significant differences from baseline values in post-hoc comparisons following one-way ANOVA.
II Experiments in Humans
All procedures were readily acceptable to all participants without inducing any perceived masticatory fatigue or other discomforts, with the exception of a slight nuisance due to salivation in a few subjects. Figure 2 describes the time course of the mean values of SBP, DBP, MABP and HR. As shown in the figure, the steep decrease in SBP and MABP in ME-treated subjects and a reduction in HR observed in both groups. The results of one-way ANOVA for repeated measures revealed a significant decrease in SBP (P<0.001), MABP (P=0.013) and HR (P<0.001). At baseline, SBP was 153±11.6 mmHg; DBP 95 ± 10.3 mmHg, MABP 114±8.1 mmHg and HR 76 ±12.8 bpm. The BP decline after mandibular extension reached a nadir at about 50-90 min, when SBP was 137±9.9 mmHg, DBP 88 ± 9.2 mmHg and MABP 104 ± 8.5 mmHg. HR values steadily decreased. Post-hoc comparisons that attained statistical significance are reported in (Figure 2) by asterisks. In controls, systolic, diastolic, and mean arterial blood pressure values oscillated during the observation period within a limited range corresponding to 145-151 mmHg for SBP, 93-95 mmHg for DBP, and 110-113 mmHg for MABP.
Discussion
This study carried out on elderly SHR and adult hypertensive humans, shows that a passive sub-maximal opening of the mouth causes a significant reduction in arterial blood pressure. These findings are in line with those obtained in previous studies in which a similar phenomenon was observed in normotensive rats and in young normotensive humans [6, 7, 10]. In the latter case, the hypotensive effect recorded was much lower since a ME-induced SBP reduction of 4-5 mmHg was observed, while in the present study, a decrease of about 15 mmHg was recorded in older hypertensive subjects [9, 10]. Similarly, in the normotensive subjects of the former study, the decline in DBP was less marked, with a non-significant reduction of about 3 mmHg, while in the hypertensive subjects of the present study, ME induced a still non-significant decrease of about 6 mmHg [9, 10]. The temporal course of blood pressure during this experiment is very similar to that previously observed in young normotensive subjects, with the blood pressure decline starting about 20 minutes after the administration of ME in both studies and attaining a nadir between 50 and 90 minutes for SBP and after 70 minutes for DBP. In all cases, a similar linear reduction in HR has been observed, with a statistically significant decrease compared to baseline starting from the 80th minute.
The hypotensive response following ME observed in hypertensive humans is paralleled by that observed in the elderly SHR rats, where an even greater and more prolonged effect was observed, with a significant reduction of up to 50 mmHg in SBP, 40 mmHg in DBP and 40 mmHg in MABP. In previous studies only the MABP was recorded, and a decrease with respect to controls was observed, which was of 30 mmHg in non-elderly SHR rats and of 15 mmHg in non-elderly normotensive rats [8, 11]. The hypotensive effect observed in this study corresponds to a decrease of about 40 mmHg in MABP, which is by about 10 mmHg larger than that observed in non-elderly SHR rats and by about 25 mmHg larger than that observed in non-elderly normotensive rats [8, 11]. These observations suggest a relation between baseline BP and hypotensive response so that the effects of ME appear greater in the hypertensive condition.
It may be interesting to note that the ME-induced hypotensive response recalls for various aspects the so-called post-exercise hypotension (PEH). This phenomenon consists of a reduction in blood pressure that occurs after carrying out mild and moderate physical activities [14, 15]. Several features of PEH are remarkably similar to those observed after ME, like the fact that exercises must have a duration of at least 10 minutes to be effective, that PEH is a prolonged phenomenon and can last up to two hours, and that the amplitude of the PEH response varies between 8 and 10 mmHg for SBP and from 3 to 5 mm Hg for DBP in normotensive subjects and is more pronounced in rats than in humans [14-16]. All these aspects find a similarity in what we have observed in previous studies on ME [6-8]. In addition, in humans, PEH is known to be larger in hypertensive than in normotensive subjects, in accordance with the results of the present study [17].
However, ME differs from the models of physical activity patterns in that an increase of HR or BP levels is observed at the onset of physical activity, while such an increase was not observed during ME. For this reason, we think that ME should probably be best viewed as a kind of passive stretching activity. It is interesting to note that the muscle stimulated by ME treatment is the masseter, which is not such a large muscle, but, in any case, it is a powerful muscle that can exert considerable contraction force and is finely innervated [18, 19]. It has been observed that mild physical activities such as static stretching in other muscular districts are sufficient to induce blood pressure reduction, without an increase during the exercise, analogously to what was observed with ME [20]. Kruse and co-workers showed that in healthy young male subjects, a single static stretching event of the triceps muscle was followed by a significant reduction of DBP by 4 mmHg and that this effect persisted for up to 10 minutes after the end of stretching; for SBP the reduction was 3-7 mmHg even if it did not attain statistical significance [21]. Moreover, Wong and Figueroa found that in postmenopausal women, 8 weeks of training through stretching of the major muscle groups resulted in a significant reduction in both SBP and DBP [22].
The physiological mechanisms underlying the ME-induced hypotensive effect are still unclear and the structures involved in the afferent arc of this reflection are not yet known. The fact that in the normotensive rat, the cardiovascular effects of the mandibular extension were found to be abolished by bilateral peripheral trigeminal section indicates a role of the cranial nerve as the afferent limb of this response and suggests that this effect is part of the so-called trigemino-cardiac reflexes [2-5]. ME treatment has been found to modulate gene expression and protein levels of the components of the renin-angiotensin system in different cerebral cortex areas, and to be able to increase nitric oxide induced endothelial vasodilation of the cerebral arterioles in different cerebral areas [7, 8, 23, 24]. On this connection, it is worth stressing that nitric oxide seems to play an important role also in the effect of static stretching. This suggesting by a recent study on elderly rats in which repeated stretching for 4 weeks improves NO-dependent endothelial vasodilation and angiogenesis in the vessels of the stretched skeletal muscles [25].
It is acknowledged that the validity of our result in humans may be limited by the small sample size, particularly of the control group, which was due to the difficulty of recruiting subjects who met the stringent criteria set by the experimental protocol (see above). However, our comparative study still provides clear evidence that a sub-maximal passive masseter stretching can induce a prolonged reduction in blood pressure, particularly in systolic pressure, not only in normotensive but even more in hypertensive subjects. All this opens up interesting perspectives to understand how techniques of passive ME could find application in the medical field as added non-pharmacological aids in the treatment of mild to moderate arterial hypertension.
Ethical Approval
Experiments on rats were carried out in agreement with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Pisa and the Ministry of Health (Permit Number: 157/2017-PR). Hypertensive human subjects were recruited after their first cardiologic visit at the Outpatient Clinic of the Fondazione G. Monasterio, Pisa. Informed consent was obtained from all subjects and the study was approved by the local Ethical Committee (Comitato per lo studio del farmaco sull’uomo, Azienda Ospedaliera di Pisa, Protocol number 3654).
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 01, Mar 2021Accepted: Tue 16, Mar 2021
Published: Mon 19, Apr 2021
Copyright
© 2023 Cristina Del Seppia. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2021.01.06
Author Info
Cristina Del Seppia Giuseppe Federighi Enza Fommei Sergio Ghione Rossana Scuri
Corresponding Author
Cristina Del SeppiaInstitute of Clinical Physiology, National Council of Research (CNR), Pisa, Italy
Figures & Tables
Table 1: Mean ± SEM values of arterial blood pressure.
|
N =6 |
SBP |
DBP |
MEAN |
HR |
|
Awake rats |
200±6.5 |
173±8.9 |
177±8.4 |
484±25.7 |
|
Anaesthetized rats |
197±7.0 |
177±8.8 |
182±7.5 |
532±13.5 |
References
1.
Kumada M, Dampney RA, Reis DJ
(1977) The trigeminal depressor response: a novel vasodepressor response
originating from the trigeminal system. Brain Res 119: 305-326. [Crossref]
2.
Chowdhury T,
Schaller B (2017) Chronic Trigemino-Cardiac Reflex: An Underestimated Truth. Front
Neurol 8: 22. [Crossref]
3.
Cornelius JF, Sadr Eshkevari P, Arasho BD,
Sandu N, Spiriev T et al. (2010) The trigemino-cardiac reflex in adults: own
experience. Expert Rev Cardiovasc Ther 8: 895-898. [Crossref]
4. Schaller B (2004) Trigeminocardiac
reflex. A clinical phenomenon or a new physiological entity? J Neurol
251: 658-665. [Crossref]
5. Schaller B, Cornelius JF, Prabhakar
H, Koerbel A, Gnanalingham K et al. (2009) The trigemino-cardiac reflex: an
update of the current knowledge; Trigemino-Cardiac Reflex Examination Group
(TCREG). J Neurosurg Anesthesiol 21: 187-195. [Crossref]
6. Lapi D, Colantuoni A, Del Seppia C,
Ghione S, Tonlorenzi D et al. (2013) Persistent effects after trigeminal nerve
proprioceptive stimulation by mandibular extension on rat blood pressure, heart
rate and pial microcirculation. Arch Ital Biol 151: 11-23. [Crossref]
7. Lapi D, Federighi G, Fantozzi MP,
Seppia CD, Ghione S et al. (2014) Trigeminocardiac reflex by mandibular
extension on rat pial microcirculation: role of nitric oxide. PLoS One
9: e115767. [Crossref]
8. Lapi D, Varanini M, Colantuoni A,
Seppia CD, Ghione S et al. (2017) Repeated Mandibular Extension in Rat: A
Procedure to Modulate the Cerebral Arteriolar Tone. Front Physiol 8:
625. [Crossref]
9. Del Seppia C, Ghione S, Foresi P,
Fommei E, Lapi D et al. (2016) Further evidence of a prolonged hypotensive and
a bradycardic effect after mandibular extension in normal volunteers. Arch Ital Biol 154: 143-150. [Crossref]
10. Del Seppia C, Ghione S, Foresi P,
Lapi D, Fommei E et al. (2017) Evidence in the human of a hypotensive and a
bradycardic effect after mouth opening maintained for 10 min. Eur J Appl
Physiol 117: 1485-1491. [Crossref]
11. Del Seppia C, Lapi D, Ghione S, Federighi G, Sabatino
L et al. (2018) Evidence in hypertensive rats of hypotensive effect after mandibular
extension. Physiol Rep 6: e13911. [Crossref]
12.
Doggrell SA, Brown L (1998) Rat models of
hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39:
89-105. [Crossref]
13. Olsen MH,
Angell SY, Asma
S, Boutouyrie P, Burger D et al. (2016) A call to action and a lifecourse
strategy to address the global burden of raised blood pressure on current and
future generations: the Lancet Commission on hypertension. Lancet 388:
2665-2712. [Crossref]
14. Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano
JD et al. (2013) Beyond medications and diet: alternative approaches to
lowering blood pressure: a scientific statement from the american heart
association. Hypertension 61: 1360-1383. [Crossref]
15.
Kenney MJ, Seals DR (1993) Postexercise hypotension.
Key features, mechanisms, and clinical significance. Hypertension 22:
653-664. [Crossref]
16. MacDonald JR, MacDougall JD, Hogben
CD (2000) The effects of exercise duration on post-exercise hypotension. J
Hum Hypertension 14: 125-129. [Crossref]
17. Fagard RH (1993) Physical fitness
and blood pressure. J Hypertens Suppl 11: S47-S52. [Crossref]
18. Beck TW, Housh TJ, Cramer TJ, Weir
JP, Johnson GO et al. (2005) Mechanomyographic amplitude and frequency
responses during dynamic muscle actions: a comprehensive review. Biomed Eng Online 4: 67. [Crossref]
19. Giaconi E, Deriu F, Tolu E, Cuccurazzu B, Yates BJ et al. (2006)
Transneuronal Tracing of Vestibulo-Trigeminal Pathways Innervating the Masseter
Muscle in the Rat. Exp Brain Res 171: 330-339. [Crossref]
20. Kruse NT, Scheuermann BW (2017)
Cardiovascular Responses to Skeletal Muscle Stretching:‘‘Stretching’’ the Truth
or a New Exercise Paradigm for Cardiovascular Medicine? Sports Med 47:
2507-2520. [Crossref]
21.
Kruse NT, Silette CR, Scheuermann BW (2016) Influence
of passive stretch on muscle blood flow, oxygenation and central cardiovascular
responses in healthy young males. Am J Physiol Heart Circ Physiol 310:
H1210- H1221. [Crossref]
22. Wong A, Figueroa A (2014) Eight
weeks of stretching training reduces aortic wave reflection magnitude and blood
pressure in obese postmenopausal women. J Hum Hypertens 28:
246-250. [Crossref]
23. Sabatino L, Costagli C, Lapi D, Seppia CD, Federighi G et al. (2018) Renin-Angiotensin system responds to prolonged
hypotensive effect induced by mandibular extension in spontaneously hypertensive
rats. Front Physiol 9: 1613. [Crossref]
24.
Lapi D, Varanini M, Galasso L, Maro MD, Federighi G et
al. (2019) Effects of Mandibular Extension on Pial Arteriolar Diameter Changes
in Glucocorticoid-Induced Hypertensive Rats. Front Physiol 10: 3. [Crossref]
25. Hotta K, Behnke BJ, Arjmandi B, Ghosh P, Chen B et al.
(2018) Daily muscle stretching enhances blood flow, endothelial function,
capillarity, vascular volume and connectivity in aged skeletal muscle. J
Physiol 596: 1903-1917. [Crossref]
