Journals
Heart rate regulation in patients with anorexia nervosa
A B S T R A C T
Background and Aims: To examine the effects of starvation and nutritional refeeding on heart rate variability (HRV) and heart rate in adolescents with Anorexia Nervosa.
Method: For this publication we illustrate 3 precedential cases to explain our model of heart rate regulation in patients with Anorexia Nervosa.
Results: The linear relationship between the range of normal RR-intervals, global heart rate variability (SDNN) and mean heart rate is explainable by the slope of the diastolic depolarization of sinus node cells, regulated by the funny channels. We demonstrate the relationship between caloric intake, heart rate and heart rate variability which is better explained by the regulation of the funny channels than by the autonomic nervous system. However, the impaired heart rate regulation induces dysautonomia, demonstrated in two patients who develop postural orthostatic tachycardia syndrome (POTS). Based upon this model we explain the therapeutics effects of beta blockers, midodrine and ivabradine in these patients.
Discussion: Our model of heart rate regulation in patients with Anorexia Nervosa explains dysautonomia in these patients and the effect of refeeding on heart rate regulation. Treatment of the impaired heart rate regulation in these patients with beta blockers, midodrine and ivrabradine can improve the often-unsatisfactory therapy results.
K E Y W O R D S
Adolescent, anorexia nervosa, autonomic nervous system, heart rate, funny channel, postural orthostatic tachycardia syndrome, ivabradine
Introduction
Anorexia nervosa (AN) has the highest mortality rate among psychiatric disorders that is in part related to cardiovascular events [1]. Analysis of heart rate variability (HRV) is a promising tool for a better understanding of the pathophysiology of anorexia nervosa and improvement of therapy. However, to date the results of previous HRV studies in patients with AN are contradictory, which may be due to inconsistent HRV measurements that ignore the stage of refeeding [2]. Most studies found a reduced heart rate and enhanced HRV in patients with AN but the reason remained unclear: Most authors anticipate a higher vagus activity as the reason for lower heart rate. However current research focused on heart rate regulation in cardiac disease or sports found other explanations: Yaniv et al. suggested that ‘deficits in intrinsic regulatory properties of pacemaker cells during cardiac disease can affect heart rate and HRV’ [3]. Boyett et al also hypothesized that remodeling of the sinoatrial node and ‘a training-induced alteration in the electrophysiology of the sinoatrial node could reset both the resting and maximal HR at a lower level’ and explain training-induced bradycardia. Especially downregulation of Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN4) could be responsible for chronotropic incompetence and bradycardia in AN [4].
In 2005, our department began to perform at least one 24-h electrocardiogram (ECG) for each patient with AN admitted to the department of pediatrics for in-hospital refeeding. Since then, in accordance with companion studies, we have noted that most adolescents with AN have a low HR and high HRV parameters during the state of starvation [5]. Moreover, we observed a significant increase of heart rates and decrease of HRV during refeeding which could explain the discomfort due to this “autonomic switch” and the massive resistance against this therapy from the patients [6]. Based on this pathophysiological understanding, we changed our therapeutic concepts and can significantly improve weight gain during in-hospital refeeding [7]. Within the last 13 years and more than 100 Holter ECG’s in patients with AN that include longtime follow up data, we increasingly began to doubt our primary assumption that the HRV changes are caused solely by the autonomic nervous system. In this paper we try to publish our current hypothesis to explain the pathophysiology of heart rate regulation in patients with anorexia nervosa and the promising therapeutic consequences in three extraordinary cases.
Figure 1: Body Mass Index of a girl with anorexia nervosa, starting suddenly at the age of 12 years. In hospital refeeding in three specialized clinics for nearly 2 years. Successful refeeding in our clinic with an age of 15 years. Outpatient treatment for further 3 years (Case 1)
Method
Heart Rate Variability Analysis (HRV)
HRV measurements are based on the RR intervals of normal QRS complexes (NN intervals) and are obtained using 24-hour Holter ECG. The most used HRV indices can be differentiated into time-domain parameters and frequency domain parameters (Table). Time domain parameters include standard deviation of NN intervals (SDNN, measured in ms), which reflects the general variability of heart rate influenced by the ANS, endocrine, and thermoregulatory mechanisms during the period of recording; pNN50 (%), which qualifies percent of R-R intervals differing more than 50 ms from the preceding one and mainly reflects parasympathetic influences and is therefore correlated with HF power; and Root Mean Square of the Successive Differences (RMSSD, measured in ms), which reflects vagal tone.
Frequency parameters include ultra-low frequency power (ULF; below 0.0033 Hz; ms2), whose physiological correlate is unknown but may reflect circadian fluctuations, body temperature, and metabolism; very low frequency power (VLF; 0.0033–0.04 Hz; ms2), which may represent thermoregulation/resting energy expenditure, kidney functioning and hormonal mechanisms; low-frequency power (LF; 0.04–0.15 Hz; ms2), which is mediated by sympathetic tone and constitutes an index of baroreflex-function but may represent both sympathetic and parasympathetic activity; high-frequency power (HF; 0.15–0.40 Hz; ms2), which represents sinus arrhythmia and is mediated by alternating levels of parasympathetic tone; LF/HF ratio, which controversially represents the balance between sympathetic and parasympathetic tone; and total power (TP; ms2), which measures the total variance in HRV. For this publication we introduce the parameter NN-range that is read from the NN-histograms (Figure 7) and not simply calculated as difference between the highest and lowest heart rate to be more resistant against outliers.
Table 1: Definitions of Variables of Heart Rate Variability
|
Variable |
Unit |
Description |
|
|
|
Time domain measures |
|
Mean NN |
ms |
Mean value of all normal RR intervals during 24 h |
|
SDNN |
ms |
Standard deviation of all NN intervals |
|
pNN50 |
% |
Number of pairs of adjacent NN intervals differing by more than 50ms divided by the total number of all NN intervals |
|
rMSSD |
ms |
The square root of the mean of the sum of the squares of differences between adjacent NN intervals |
|
|
|
Frequency domain measures |
|
Total Power |
ms2 |
Heart rate power spectrum between 0,003 and 0,4 Hz |
|
VLF |
ms2 |
Very low frequency power spectrum between 0,003 and 0,04 Hz |
|
LF |
ms2 |
Low frequency power spectrum between 0,04 and 0,15 Hz |
|
HF LF/HF ratio |
ms2 |
High frequency power spectrum between 0,15 and 0,4 Hz Ratio of low to high frequency power |
Patients
Case 1
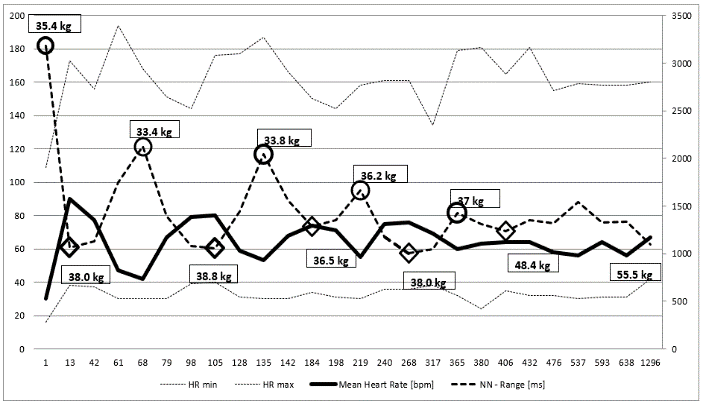
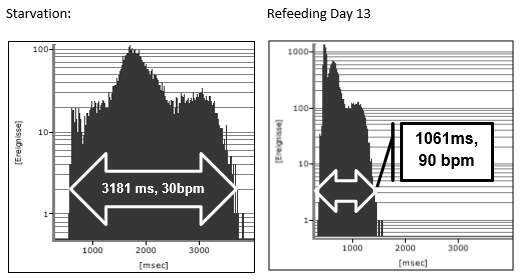
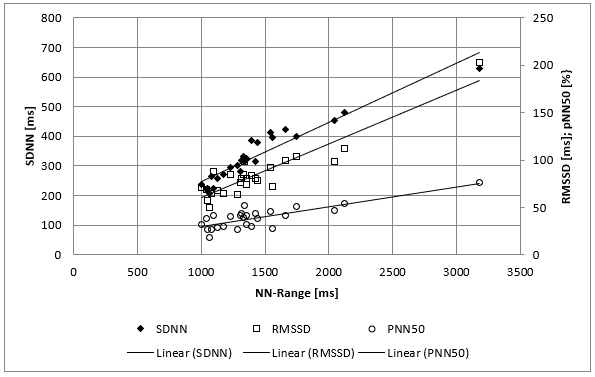
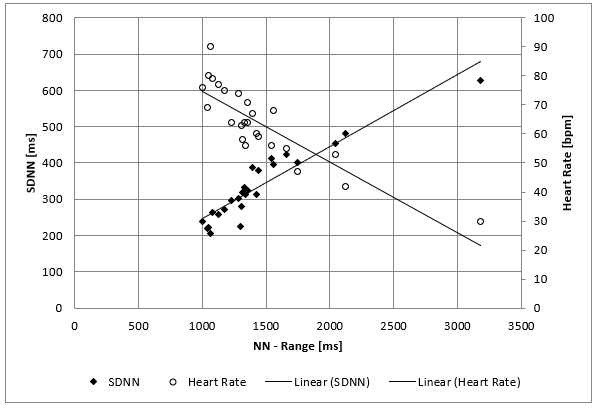
The clinical course of this girl is illustrated by the body percentiles in (Figure 1). She lost 24 kg bodyweight within one summer and failed to improve her condition despite long hospital stays in three specialist clinics of pediatric psychiatry including nasogastric tube feeding. Some days after the last hospital discharge, we admitted the girl for in hospital refeeding in a very bad condition with a mean heart rate of 30/min and bradycardias up to 19/min at night. We need 4 hospital stays within one year as shown in (Figure 2) until the girl accept nasogastric tube feeding with a significant weight gain of 10 kg over three months. During each refeeding step we observed the same changes of heart rate and HRV as illustrated in (Figure 2) and (Figure 3): The NN-histograms (Figure 3) show an impressive NN-range of 551 to 3732 ms at the first admission that decrease to 365 to 1426 ms after only twelve days of refeeding. The most important issue of heart rate regulation in this girl is the discordant change of mean heart rate and NN-range that not depends on the differences between the highest and lowest heart rate (Figure 2). Furthermore, it is the NN-range and mean heart rate that predict global heart rate variability indicated by the parameter SDNN (Figure 4) and Total Power (Figure 5). The increase of SDNN and Total Power is paralleled by all other time domain and frequency domain parameters (Figure 4 & 5). We speculate that the increases of the vagus parameters rMSSD, pNN50 and high frequency power are the consequence of the higher NN range and not solely of a higher vagus activity. This important issue is supported by the impressive linear relationship of NN-range with the mean heart rate and SDNN as shown in (Figure 6).
Figure 2: Holter ECG monitoring of heart rate over nearly four years of a girl with anorexia nervosa (Case 1). Body weight at relapse are shown in the circles, body weight after refeeding is shown in the rhombus. Mean heart rate increase and the heart rate range (NN-range) decrease during refeeding.
Figure 3: NN-histogramm of two 24-hour Holter ECG during starvation and after 13 days refeeding of a girl with anorexia nervosa (Case 1).
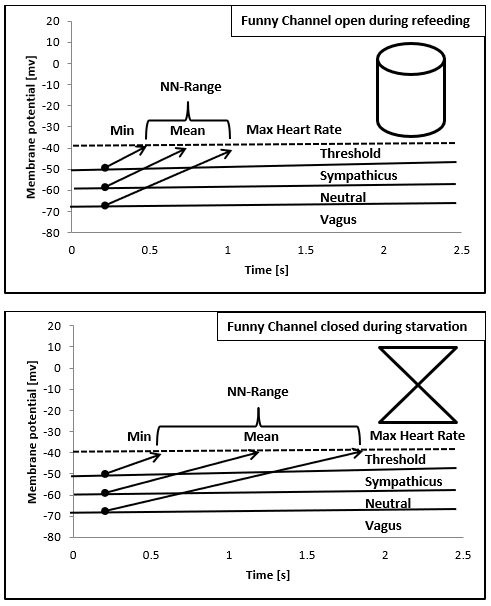
The theoretic explanation of the linear relationship between NN-range and mean heart rate can be derived from the slope of the diastolic depolarization in the sinus node that is regulated from the funny channels (Figure 7 and discussion). The global HRV parameter SDNN is the calculation of the standard deviation of NN-intervals that depends on the different NN-ranges during starvation and refeeding (Figure 3). The drift of the NN-range from 3181 ms to 1061 ms leads to the elevation of the 24 hours mean heart rate from 30/min to 90/min.
Figure 4: Mean SDNN/RMSSD/pNN50 values of 26 twenty-four hours Holter ECG’s of a girl with anorexia nervosa (Case 1) within nearly four years. There is a linear relationship between the range of normal RR Interval (NN-Range) and the heart rate variability (Time domaine analysis).
Case 2 & 3: The POTS Problem
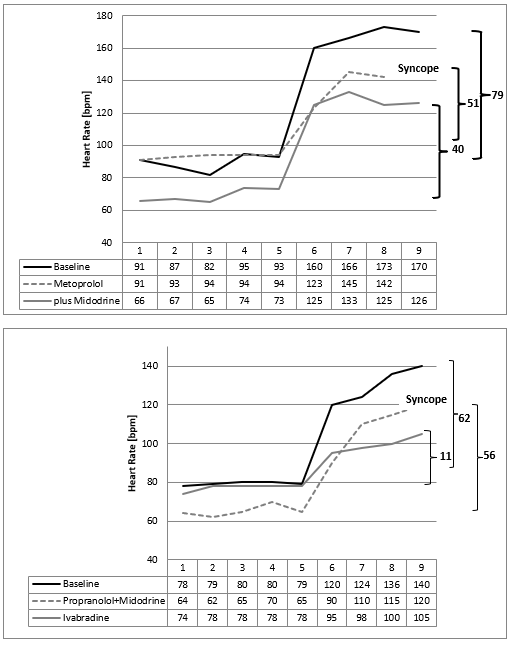
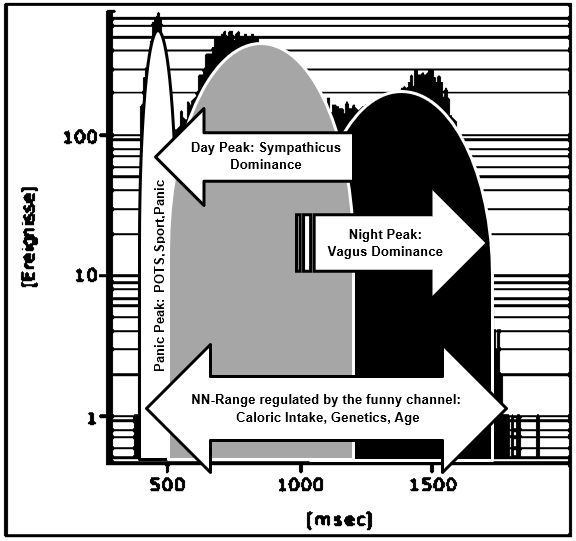
There are some mysteries about the high heart rates in the NN-histograms during refeeding (Figure 3 & 10). We speculate about increasing sports that is not forbidden in our clinic during refeeding or emotional stress about the increasing bodyweight. However, these speculations were supplemented by the clinical observation of a 16-year-old boy who became bedridden during refeeding. We interpreted this condition as severe depression and started an intensive psychopharmacological therapy, which was unsuccessful. Despite lying in the bed nearly the whole day, we detect high heart rates in the Holter ECG induced by orthostasis. He had severe postural orthostatic tachycardia syndrome (POTS) in the tilt test that must be treated (Figure 8). After low dose metoprolol he collapsed at the next tilt test. But together with midodrine he recovered, and he was able to handle his daily tasks without any problems. With low dose Fluoxetine he has perfect weight gain in an outpatient setting.
Figure 5: Mean Total Power, Ultra Low Frequency, Very Low Frequency, Low Frequency and High Frequency Power values of 26 twenty-four hours Holter ECG’s of a girl with anorexia nervosa (Case 1) within nearly four years. There is a linear relationship between the range of normal RR Interval (NN-Range) and the heart rate variability (Frequency domaine analysis).
Figure 6: Mean SDNN and mean heart rate values of 26 twenty-four hours Holter ECG’s of a girl with anorexia nervosa (Case 1) within nearly four years. There is a linear relationship between the range of normal RR Interval (NN-Range), global the heart rate variability (SDNN) and the mean heart rate.
The next girl who developed POTS during refeeding benefits from low dose Propranolol and Midodrine, but she collapsed during the tilt test too (Figure 8). We changed to ivabradine plus midodrine and she recovered from POTS. After 6 weeks of unsuccessful refeeding her condition improved, and refeeding was successful.
Figure 7: Illustration of the relationship between mean heart rate and NN-Range based upon the diastolic depolarization of the sinus node cells regulated by the funny channel and the autonomic nervous system. The funny channel regulates the slope of diastolic depolarization; the autonomic nervous system regulates the membrane potential.
Figure 8: Tilt test of two patients with anorexia nervosa who develop postural orthostatic tachycardia syndrome during refeeding (Case 2+3) with a maximal heart rate increase after standing up (minute 5) of 79 beats /minute (Case 2) and 62 beats/minute (case 3). Beta blockers and midodrine show only a slight improvement of the heart rate increase. Ivabradine reduce the heart rate increase from 62 beats per minute to 11 beats/minute.
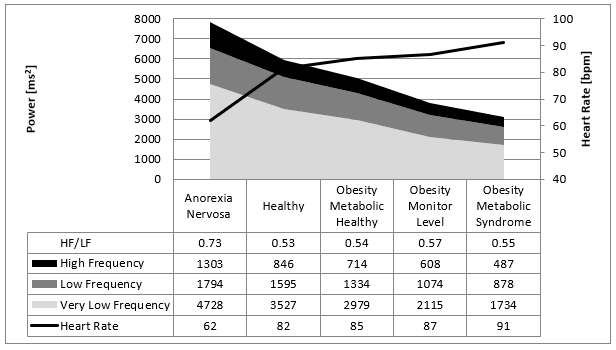
Figure 9: Overview about our data from children with eating disorders indicated by the mean values of the Fast Fourier Analysis in comparison to children with normal body weight. There is a linear relationship between caloric intake/metabolism to the heart rate variability illustrated by the frequency domaine analysis.
Discussion
Caloric intake has a high impact on heart rate and HRV. Our recently published data are summarized in (Figure 9): Low caloric intake decrease heart rate and increase HRV [5, 6]. High caloric intake increases heart rate and decrease HRV. These data are important to understand the cardiovascular complications of nutritional disorders [8]. Taken together these cardiovascular complications due to obesity and anorexia nervosa are one of the biggest challenges for the health systems of industrialized countries. However, despite the significant statically relationship between caloric intake and heart rate regulation, the pathophysiological interpretation remains contradictory. We now discuss our clinical cases of children with anorexia nervosa who becomes precedential cases for our model of heart rate regulation in patients with anorexia nervosa:
Case 1
The first clinical case impressed by the high reproducibility and very high range of heart rate changes in 4 hospital stays for refeeding in a girl with supposedly resistant anorexia nervosa therapy, who had frequent relapses of starvation despite intensive in hospital psychotherapy after a very short massive weight loss of 24 kg within only 8 weeks. Beginning in the second year of her disease, we perform 26 Holter ECG’s during refeeding and a longtime follow up of 3.5 years. Based on the compulsive personality of the patient, the examinations were conducted under very standardized conditions, including a 1-hour sports exercise a day. These highly standardized conditions of investigation reveal the highly significant linear relationships of the heart rate parameters with HRV, summarized in (Figure 6). Ad admission in our hospital after longtime in Hospital refeeding in other clinics she nearly reaches the extremes of this linear relationship with an NN-range from 3181 ms and a mean heart rate of 30/min. Within only 12 days of refeeding she has tripled her mean heart rate and reduced the NN-range by a factor of 3. She didn’t tolerate this autonomic switch and relapse despite small changes of bodyweight. Holter ECG monitoring revealed each relapse that was treated with an immediate refeeding intervention (Figure 2). After one year she agreed to a nasogastric refeeding up to a weight of 48 kg and need no further hospitalization for the next 2.5 years. She exactly eats 1500kcal/day and performs one hour jogging the day. Spontaneously despite unchanging calories and sports, her weight gain increases up to the starting body weight of 55,5 kg five years ago. The girl isn’t healthy, but able to handle her daily tasks. It is her extreme course that stimulates our efforts to smooth the autonomic switch during refeeding in other patients. The improved clinical results of our current refeeding protocol are published recently [8].
Case 2 & 3
These two cases demonstrate that the high heart rates during refeeding indicates a postural orthostatic tachycardia syndrome (POTS) simply diagnosed by a tilt test. As documented in several internet chat rooms (http://www.dysautonomiainternational.org), POTS seem to be more than orthostatic dysregulation. We observe a high psychological stress in children who suffer from POTS and plead for a consistent treatment of this regulation disorder. Probably sudden heart rate increases are interpreted as a stress response, regardless of cause, that can reactivate e.g. a posttraumatic stress disorder. Probably we must smooth these sudden heart rate increases (the “panic peak” in the NN-histogram Figure 10) that the soul may differentiate between panics and daily life situations. Patients with anorexia nervosa prevent these heart rate increases by a physiological hunger reaction. Based upon this concept, the beta bocker propranolol was successfully used in patients with posttraumatic stress disorder to smooth heart rate increases during therapy [9]. In our patients with POTS during refeeding, we had to learn that beta blockers improve reflex tachycardia but must be combined with midodrine to prevent syncope. However, case 3 collapsed during tilt test despite this combination therapy. From clinical observation and based upon our pathophysiological model ivabradine seems to be the therapy of choice to treat POTS during refeeding in patients with anorexia nervosa (Figure 8).
Figure 10: Interpretation of the NN-histogramm of the girl with anorexia nervosa during refeeding (Case 1). The first peak with high heart rates is related to physical and emotional exercise but also to the postural orthostatic tachycardia syndrome. The second peak illustrates heart rates during daily life and the third peak at sleep.
Our pathophysiological model of heart rate regulation in patients with anorexia nervosa
As others we discussed our data based upon the autonomic balance between the vagus and sympathicus: Low caloric intake increases vagus activity (higher RMSSD and higher high frequency power) and decrease heart rate [6]. High caloric intake reduces vagus acivity (lower RMSSD and lower high frequency power) and increase heart rate. However, as shown in (Figure 9) the ratio between high and low frequency power remained unchanged that counts for an unchanged sympathico-vagal balance in the caloric intake induced shifts of heart rate regulation. Y Yaniv and EG Lakatta recently introduce the double-clock concept of sinus node heart rate regulation based upon animal and cell culture data: On the sinus node cellular level it is not only the β-receptor and acethyl choline receptor who regulate the heart rate but also the funny channel that regulate the diastolic depolarization and heart rate [10]. Genetic studies had shown the important role of the underlying HCN 4 gene that regulate heart rate and HRV [11].
As shown in (Figure 7) heart regulation in our first case may be explained by the regulatory properties of the funny channel on diastolic depolarization: Starvation seems to block the funny channel with the mathematical consequence of a lower heart rate and higher NN-range. Refeeding open the funny channel and fasten diastolic depolarization up to the threshold that leads to higher heart rates and a smaller NN-range. The NN-histograms show a left ward shift to higher heart rates during refeeding. The day and night peaks were added by a third peak after refeeding with high heart rates that include sports, POTS and emotional stress. Within the NN-range autonomic regulation remains preserved that explained the vagus dominance during starvation due to the right ward shift of the NN-histogram. The interpretation of a real-life histogram is illustrated in (Figure 10). We call the new peak with high heart rates during refeeding the “panic peak”. Based upon this model the therapeutic consequences become clear: If the “panic peak” is caused by a POTS, we should treat according the current standards of pharmacotherapy: beta-blocker, midodrine, ivabradine, fludrocortisone. POTS treatment should be individualized to the underlying pathophysiology [12-15]. If POTS is related to a funny channel induced left ward shift of the NN-histogram during refeeding ivabradine seems to be the therapy of choice. This must be proven by prospective studies as well as the incidence of POTS during refeeding. Deconditioning is a major cause of orthostatic intolerance and POTS [16]. Deconditioning was used as an unproven therapy during refeeding in patients with anorexia nervosa that should be avoided to prevent POTS. Controlled exercise may use to improve POTS symptoms [17].
Biology of the interplay between caloric intake and heart rate regulation
In the hierarchical brain-heart circadian clock system sinus node cell function is the end effector of heart rate regulation [18]. A vital prerequisite for life is a balanced energy balance that is disturbed in patients with anorexia nervosa. In mammals, for which heart rate is a key determinant of cardiac energy demand, AMPK functions in an organ-specific manner seems to maintain cardiac energy homeostasis and determines cardiac physiological adaptation to caloric intake by modulating intrinsic sinoatrial cell behavior probably via HCN4 protein expression [19]. Our data clearly support the dominance of the intrinsic heart regulation via sinus node regulation over autonomic regulation in anorexia nervosa. In the same sense sinus bradycardia in athletes is an adaption of the intrinsic heart rate to physical strain to maintain energy balance [4]. Probably the age-related decrease of HRV is the consequence of a lower intrinsic heart rate and not solely a lower vagus activity [20].
Conflicts of Interest
There are no conflicts of interest to declare. All study participants provided informed consent, and the study design was approved by the appropriate ethics review board.
Funding
We thank the “Blaschek foundation” who supports us by covering the publication fees for this research project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 05, Mar 2019Accepted: Fri 22, Mar 2019
Published: Mon 29, Apr 2019
Copyright
© 2023 Reiner Buchhorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.PDR.2019.01.003
Author Info
Baumann C Reiner Buchhorn Willaschek C
Corresponding Author
Reiner BuchhornCaritas-Krankenhaus Bad Mergentheim, Department of Pediatrics, Uhlandstraße 7, Bad Mergentheim, Germany
Figures & Tables
Table 1: Definitions of Variables of Heart Rate Variability
|
Variable |
Unit |
Description |
|
|
|
Time domain measures |
|
Mean NN |
ms |
Mean value of all normal RR intervals during 24 h |
|
SDNN |
ms |
Standard deviation of all NN intervals |
|
pNN50 |
% |
Number of pairs of adjacent NN intervals differing by more than 50ms divided by the total number of all NN intervals |
|
rMSSD |
ms |
The square root of the mean of the sum of the squares of differences between adjacent NN intervals |
|
|
|
Frequency domain measures |
|
Total Power |
ms2 |
Heart rate power spectrum between 0,003 and 0,4 Hz |
|
VLF |
ms2 |
Very low frequency power spectrum between 0,003 and 0,04 Hz |
|
LF |
ms2 |
Low frequency power spectrum between 0,04 and 0,15 Hz |
|
HF LF/HF ratio |
ms2 |
High frequency power spectrum between 0,15 and 0,4 Hz Ratio of low to high frequency power |


References
- Arcelus J, Mitchell AJ, Wales J, Nielsen S (2011) Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 68: 724-731. [Crossref]
- Mazurak N, Enck P, Muth E, Teufel M, Zipfel S (2011) Heart rate variability as a measure of cardiac autonomic function in anorexia nervosa: a review of the literature. Eur Eat Disord Rev 19: 87-99. [Crossref]
- Yaniv Y, Lyashkov AE, Lakatta EG (2014) Impaired signaling intrinsic to sinoatrial node pacemaker cells affects heart rate variability during cardiac disease. J Clin Trials 4. [Crossref]
- Boyett MR, Wang Y, Nakao S, Ariyaratnam J, Hart G, et al. (2017) Exercise training-induced bradycardia is caused by changes in intrinsic sinus node function. J Appl Physiol (1985) [Crossref]
- Dippacher S, Willaschek C, Buchhorn R (2014) Different nutritional states and autonomic imbalance in childhood. Eur J Clin Nutr 68: 1271-1273. [Crossref]
- Buchhorn R HF, Meint S, Willaschek C (2016) The Impact of Nutrition on the Autonomic Nervous System. Int J Food Nutr Sci 3: 1-16.
- Baumann C WC, Kertess-Szlaninka T, Lang J, Buchhorn R (2017) Implementing high energy liquid nutrition, omega-3 fatty acids and nutritional supplements for the treatment of anorexia nervosa. J Pediatric Health Nutr 1: 1-12.
- Thayer JF, Lane RD (2007) The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 74: 224-242. [Crossref]
- Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J et al. (2018) Reduction of PTSD Symptoms with Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry 175: 427-433. [Crossref]
- Yaniv Y, Lakatta EG, Maltsev VA (2015) From two competing oscillators to one coupled-clock pacemaker cell system. Front Physiol 6: 28. [Crossref]
- Nolte IM, Munoz ML, Tragante V, Amare AT, Jansen R et al. (2017) Genetic loci associated with heart rate variability and their effects on cardiac disease risk. Nat Commun 8: 15805. [Crossref]
- Miller AJ, Raj SR (2018) Pharmacotherapy for postural tachycardia syndrome. Auton Neurosci 215: 28-36. [Crossref]
- Wells R, Elliott AD, Mahajan R, Page A, Iodice V et al. (2018) Efficacy of Therapies for Postural Tachycardia Syndrome: A Systematic Review and Meta-analysis. Mayo Clin Proc 93: 1043-1053. [Crossref]
- Delle Donne G, Roses Noguer F, Till J, Salukhe T, Prasad SK et al. (2018) Ivabradine in Postural Orthostatic Tachycardia Syndrome: Preliminary Experience in Children. Am J Cardiovasc Drugs 18: 59-63. [Crossref]
- Xu WR, Jin HF, Du JB (2016) Pathogenesis and Individualized Treatment for Postural Tachycardia Syndrome in Children. Chin Med J (Engl) 129: 2241-2245. [Crossref]
- Parsaik A, Allison TG, Singer W, Sletten DM, Joyner MJ et al. (2012) Deconditioning in patients with orthostatic intolerance. Neurology 79: 1435-1439. [Crossref]
- Fu Q, Levine BD (2018) Exercise and non-pharmacological treatment of POTS. Auton Neurosci 215: 20-27. [Crossref]
- Yaniv Y, Lakatta EG (2015) The end effector of circadian heart rate variation: the sinoatrial node pacemaker cell. BMB Rep 48: 677-684. [Crossref]
- Yavari A, Bellahcene M, Bucchi A, Sirenko S, Pinter K et al. (2017) Mammalian gamma2 AMPK regulates intrinsic heart rate. Nat Commun 8: 1258. [Crossref]
- Yaniv Y, Ahmet I, Tsutsui K, Behar J, Moen JM et al. (2016) Deterioration of autonomic neuronal receptor signaling and mechanisms intrinsic to heart pacemaker cells contribute to age-associated alterations in heart rate variability in vivo. Aging cell 15: 716-724. [Crossref]
