Giant Hematoma of Rectus Abdominis Muscle in a Patient with Schistosomiasis without Periportal Abnormalities
A B S T R A C T
A 60-year-old patient came to the IIER due to abdominal pain for 2 days, associated with flu with cough. The patient treated schistosomiasis twice without effects. During physical examination and complementary exams, she presented ecchymosis in the midline of the abdomen, normal platelet level, giant hematoma in the rectus abdominais muscle without periportal changes. A careful retrospective analysis shows inconsistency between the initial platelet levels and the severe progression of the clinical. The investigation and confirmation of thrombocytopenia is very important, and some medical center recommend manual counting through blood smears. Prospective studies are needed to know how platelet measurements should be performed in schistosomiasis.
Keywords
Schistosomiasis mansoni, rectus abdominis, platelet function tests, shock, hemorrhagic
Introduction
Rectus abdominis muscle hematoma (RSH: Rectus Sheath Hematoma) is defined as an accumulation of blood in the rectus muscle sheath from the rupture of one or more branches of the inferior epigastric artery (AEI) [1]. Except in cases of anatomical variation, the AEI appears as a branch of external iliac artery with the function of perfusing the rectus abdominais muscle (RAM) together with the superior epigastric artery that comes from the internal thoracic artery [1, 2]. The anatomial study of RSH shows absense of a posterior sheath in its distal third delimited by the arcuate line (AL). Proximal to the AL, the branches of AEI are fixed, which favours vascular injury due to movement of the distal portion of the RAM [2].
In addition of clinical signs and non-specific simptoms like abdominal pain and ecchymosis, patients with RSH present some particular characteristics, as shown in (Table 1) [1, 3]. Some epidemiological conditions, such as elderly women, can predispose the RSH. Table 2 presents some risk factors to RSH [4]. For a long time, the diagnosis of RSH has been performed through computerized tomography, allowing classification and to make a clinical decision to this condition in three stages (Table 3). This method cannot measure precisely the size of the hematoma [5, 6]. Most patients do not need surgical treatment, but those with stages II and III can present haemodynamic instability, requiring surgical intervention [7-9].
Differently from RSH, Schistosomiasis is a infectious disease defined by the presence of Schistosoma (parasitic worm) in the bloodstream [10-12]. It is believed that Schistossoma mansoni (SM) eggs secrete proteins causing an immune response (Th-1 e Th-2) in the host, leading to an eosinophilic granulomatous reaction [13, 14]. Although most patients are asymptomatic, (Table 4) shows important clinical features at different stages of the disease caused by MS [10, 11]. Eradication of adult worms in the context of newly acquired schistosomiasis usually leads to complete remission of urinary and intestinal lesions [10, 11].
Table 1: Possible clinical manifestations of RSH.
|
Hypovolemic shock |
|
Abdominal compartiment syndrome |
|
Carnett’s sign |
|
Fothergill’s sign |
Table 2: Risk factos to RSH.
|
Systemic arterial hypertension |
|
Liver cirrosis |
|
Peripheral obstructive pulmonary disease |
|
Pneumonia |
|
Cough |
|
Anticoagulant and antiplatelet drugs |
Table 3: Classification of RSH.
|
TYPE |
CLINICAL PRESENTATION |
CONDUCT |
|
I |
Small, RAM-restricted hematoma |
Outpatient treatment. Clinical conservative management; |
|
II |
Significant hematoma restricted to the RAM, can exceed the midline |
Impatient treatment. Clinical or surgical management |
|
III |
Major hematoma with the possibility of: -Haemoperitoneum -Hematoma in the space of Retzius -Hematoma below the arcuate line |
Impatient treatment. Clinical or surgical management |
Table 4: Stages of Schistosomiasis by SM.
|
ACUTE PHASE |
CHRONIC PHASE |
|
KATAYAMA’S FEVER URTICARIA MYALGIA HEADACHE DIARRHEA EOSINOPHILIA (causing encephalitis without vasculitis) |
ABDOMINAL PAIN INAPETENCE DIARRHEA IRON DEFICIENCY ANEMIA NO HEMATIC CHANGES (no eosinophilia) NORMAL TRANSAMINASES SYMMERS’ FIBROSIS - occlusion of the portal vein (non-cirrhotic) by
collagen deposition along the stellate cells of the periportal space SPLENOMEGALY SHUNT PORTO CAVA GLOMERULOPATHY (proteinuria with nephrotic syndrome) |
Case Report
Female patient, black, 60 years old, from an endemic area for Schistosomiasis came to the emergency room of Emilio Ribas Hospital (IIER) complaining of severe abdominal pain for 1 day after severe coughing episode 2 days ago, associated with flu symptoms for 6 days. She denied headache, fever, nausea, diarrhea, constipation, urticaria or myalgia.
I Previous Treatments
i Schistosomiasis
Treated twice with Praziquantel (150 mg 8/8h) without medication side effects as abdominal pain, nausea, diarrhea, vomiting, dizziness, drowsiness, headaches and no increase in sweating.
ii Asthma and Pulmonary Hypertension
Treated with Carvedilol (12.5 mg 12/12h), Furosemide (40 mg 24/24h) and Salbutamol (100 mcg 12/12h).
On physical examination, the patient was lucid, oriented and haemodynamically stable. Abdominal examination revealed ecchymosis in the midline of approximately 18 cm in extension not adhered to deep planes, without pulsatility or fremitus, with pain on superficial palpation, negative abrupt decompression, rectal examination without alterations such as melena, positive Carnett and Fothergill signs (Figure 1).
II Laboratory Exams at the Time of Admission
Platelets: 114 mil/mm3 (normal 150 - 450 mil/mm3)
Eosinophil: 0.7% (normal: 1-5%)
AST: 40 U/L (normal < 31 U/L)
ALT: 53 U/L (normal < 45 U/L)
Prothrombin time: 17.9 / AP: 56% (normal > 70%).
Abdominal ultrasonography demonstrated splenomegaly (14 × 7.7 cm) normal sized liver. Upper digestive endoscopy demonstrated esophageal varices and esophageal candidiasis KODSI II. Patient refers endoscopic ligature eight years ago. Computed tomography (CT) of abdomen confirmed an extensive hematoma of the rectus abdominis muscle crossing the arcuate line and midline. Despite not having haemoperitoneum or blood in the pre-vesical space of Retzius, it was classified as RSH type III (Figure 2).
Figure 1: Abdominal examination at hospitalar admisson.
Figure 2: Axial image of abdominal tomography.
In 48 hours, the patient presented significant bleeding with changes in the static (Figure 3) and dynamic physical examinations, which showed haemodynamic instability due to significant volume loss while waiting for the procedure to be performed, evolving with hypovolemic shock, requiring vasoactive drug (noradrenaline 0,22mcg/Kg/min). Indicated surgical approach through armed arteriography.
Figure 3: Expanding hematoma after 48 h.
During the surgical procedure, she was haemodynamically unstable, and embolization of the left inferior epigastric artery was performed using a 2.7F microcatheter and use of a 0.018 non-fibered coil. In 24 hours there was haemodynamic stabilization with norepinephrine suspension. The patient remained at hospital for 16 days when he was discharged with significant resorption of the hematoma and no signs of infection. The patient had no hematoma after 10 months (Figures 4 & 5).
Figure 4: Absence of hematoma.
Figure 5: Angiotomography with the presence of a metallic coil in the arterial path.
Discussion
In addition to the diseases traditionally known as etiologic diagnoses of acute abdomen, literature reviews of RSH establish a close link with spontaneous retroperitoneal hematomas (SRH), which range from coagulopathies to ruptured aortic aneurysm [15]. Patients with SRH also presents with nonspecific abdominal pain similar to RSH, but with other important clinical signs that should be investigated in patients with acute abdomen. (Table 5). Despite the ecchymosis shown in Figure 1, we did not observe the specific signs in (Table 5) in our physical exam. The reported case presented positive Carnett and Fothergill signs, which increased the specificity and sensitivity of the diagnostic hypothesis of RSH [1, 3].
Table 5: Specific clinical signs SRH.
|
SIGN |
CHARACTERISTICS |
|
Grey Turner |
Flank ecchymosis |
|
Cullen |
Periumbilical ecchymosis |
|
Stabler |
Inguinal and/or pubic hematoma |
|
Bryant |
Ecchymosis in scrotum |
|
Fox |
Inguinal ecchymosis |
Logically, all these cases of abdominal pain with suspected RSH should undergo computed tomography in order to eliminate the possibility of SRH. Table 2 lists the use of antiplatelet agents, anticoagulants or coagulation disorders as a possible etiology of RSH. The patient was not using these medications but had thrombocytopenia. The investigation and confirmation of thrombocytopenic conditions in MS is very important, and some services recommend manual counting through blood smears [16, 17].
Graph 1 demonstrates the importance of an efficient measurement of platelets in patients with SM. But it is important to confirm that the change in the correct platelet levels is not related to the stage or severity of the disease [17, 18]. In a sample of 187 patients, Medereiros et al. concluded that the level of serum platelets establishes an inverse relationship with the severity of portal fibrosis and with the diameter of the spleen [18, 19].
Graph 1: Platelets in the first four days of hospitalization.
In the case reported, the patient had significant thrombocytopenia with almost no changes on liver and spleen anatomy. Even in patients with Symmers fibrosis and who have periportal involvement, schistosomiasis does not make elevation of AST, ALT or coagulation disorders, which is consistent with the data obtained in the serum tests of the case presented [10, 11, 19]. Based on the physical examination and complementary history, it is evident that the cough associated with thrombocytopenia caused the RSH. Once the diagnosis and classification of RSH has been performed, as shown in (Table 3), doctors can choose between clinical or surgical treatment. In most cases, there is no indication for surgery even with radiological examinations characterizing type III RSH.
However, in the presence of patients with RSH with haemodynamic instability, surgical correction is imperative. The conventional surgical technique (open technique) has greater morbidity in addition to greater difficulty in resolving multiple foci of bleeding when present. Endovascular treatment with embolization is less morbid, especially in patients using vasoactive drugs, but does not allow removal of the hematoma, which, when presented in large dimensions, may not be reabsorbed by the body, and have a high potential for infection [20, 21]. Regardless of the choice of therapy in the presence of RSH it is mandatory that these patients have urgent diagnoses and have complications or recurrences identified promptly, keeping outpatient observation.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 09, Jan 2023Accepted: Mon 30, Jan 2023
Published: Mon 06, Feb 2023
Copyright
© 2023 Alexandre Sacchetti Bezerra. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2023.01.02
Author Info
Alexandre Sacchetti Bezerra Walter Moisés Tobias Braga Raphaela Ferrari Rafi Felicio Bauab Dauar Heitor Andrei Miranda de Carvalho Rodrigo Delfino Nascimento
Corresponding Author
Alexandre Sacchetti BezerraInstituto de Infectologia Emilio Ribas, Brazil
Figures & Tables
Table 1: Possible clinical manifestations of RSH.
|
Hypovolemic shock |
|
Abdominal compartiment syndrome |
|
Carnett’s sign |
|
Fothergill’s sign |
Table 2: Risk factos to RSH.
|
Systemic arterial hypertension |
|
Liver cirrosis |
|
Peripheral obstructive pulmonary disease |
|
Pneumonia |
|
Cough |
|
Anticoagulant and antiplatelet drugs |
Table 3: Classification of RSH.
|
TYPE |
CLINICAL PRESENTATION |
CONDUCT |
|
I |
Small, RAM-restricted hematoma |
Outpatient treatment. Clinical conservative management; |
|
II |
Significant hematoma restricted to the RAM, can exceed the midline |
Impatient treatment. Clinical or surgical management |
|
III |
Major hematoma with the possibility of: -Haemoperitoneum -Hematoma in the space of Retzius -Hematoma below the arcuate line |
Impatient treatment. Clinical or surgical management |
Table 4: Stages of Schistosomiasis by SM.
|
ACUTE PHASE |
CHRONIC PHASE |
|
KATAYAMA’S FEVER URTICARIA MYALGIA HEADACHE DIARRHEA EOSINOPHILIA (causing encephalitis without vasculitis) |
ABDOMINAL PAIN INAPETENCE DIARRHEA IRON DEFICIENCY ANEMIA NO HEMATIC CHANGES (no eosinophilia) NORMAL TRANSAMINASES SYMMERS’ FIBROSIS - occlusion of the portal vein (non-cirrhotic) by
collagen deposition along the stellate cells of the periportal space SPLENOMEGALY SHUNT PORTO CAVA GLOMERULOPATHY (proteinuria with nephrotic syndrome) |
Table 5: Specific clinical signs SRH.
|
SIGN |
CHARACTERISTICS |
|
Grey Turner |
Flank ecchymosis |
|
Cullen |
Periumbilical ecchymosis |
|
Stabler |
Inguinal and/or pubic hematoma |
|
Bryant |
Ecchymosis in scrotum |
|
Fox |
Inguinal ecchymosis |
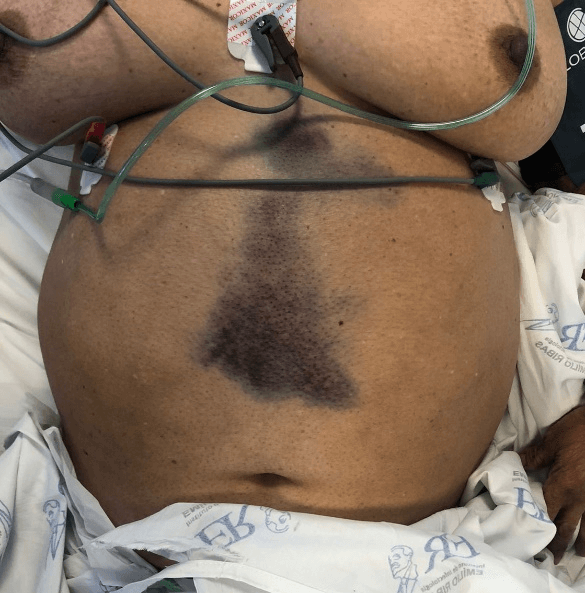





References
1. Hatjipetrou A,
Anyfantakis D, Kastanakis M (2015) Rectus sheath hematoma: a review of the
literature. Int J Surg 13: 267-271. [Crossref]
2. Mwachaka PM, Saidi
HS, Odula PO, Awori KO, Kaisha WO (2010) Locating the arcuate line of Douglas:
is it of surgical relevance? Clin Anat 23: 84-86. [Crossref]
3. Schneiderman H,
Lopetegui Lia N, Nichols J (2020) The Enduring and Practical Power of Physical
Examination: Carnett Sign. Am J Med 133: 682-684. [Crossref]
4. Kasotakis G (2014)
Retroperitoneal and rectus sheath hematomas. Surg Clin North Am 94:
71-76. [Crossref]
5. Berná JD, Garcia
Medina V, Guirao J, Garcia Medina J (1996) Rectus sheath hematoma: diagnostic
classification by CT. Abdom Imaging 21: 62-64. [Crossref]
6. Bello G, Blanco P
(2019) Giant rectus sheath hematoma. Ultrasound J 11: 13. [Crossref]
7. Warren MH,
Bhattacharya B, Maung AA, Davis KA (2020) Contemporary management of
spontaneous retroperitoneal and rectus sheath hematomas. Am J Surg 219:
707-710. [Crossref]
8. Baekgaard JS,
Eskesen TG, Lee JM, Yeh DD, Kaafarani HMA et al. (2019) Spontaneous
Retroperitoneal and Rectus Sheath Hemorrhage-Management, Risk Factors and
Outcomes. World J Surg 43: 1890-1897. [Crossref]
9. Rimola J, Perendreu
J, Falcó J, Fortuño JR, Massuet A et al. (2007) Percutaneous arterial
embolization in the management of rectus sheath hematoma. AJR Am J
Roentgenol 188: W497-W502. [Crossref]
10. Léger E, Borlase A,
Fall CB, Diouf ND, Diop SD et al. (2020) Prevalence and distribution of
schistosomiasis in human, livestock, and snail populations in northern Senegal:
a One Health epidemiological study of a multi-host system. Lancet Planet
Health 4: e330-e342. [Crossref]
11. Kamdem SD, Moyou
Somo R, Brombacher F, Nono JK (2018) Host Regulators of Liver Fibrosis During
Human Schistosomiasis. Front Immunol 9: 2781. [Crossref]
12. Carson JP, Ramm GA,
Robinson MW, McManus DP, Gobert GN (2018) Schistosome-Induced Fibrotic Disease:
The Role of Hepatic Stellate Cells. Trends Parasitol 34: 524-540. [Crossref]
13. Coutinho HM, Acosta
LP, Wu HW, McGarvey ST, Su L et al. (2007) Th2 cytokines are associated with
persistent hepatic fibrosis in human Schistosoma japonicum infection. J
Infect Dis 195: 288-295. [Crossref]
14. Kullberg MC, Pearce
EJ, Hieny SE, Sher A, Berzofsky JA (1992) Infection with Schistosoma mansoni
alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol
148: 3264-3270. [Crossref]
15. Sahu KK, Mishra AK,
Lal A, George SV, Siddiqui AD (2020) Clinical spectrum, risk factors,
management and outcome of patients with retroperitoneal hematoma: a
retrospective analysis of 3-year experience. Expert Rev Hematol 13:
545-555. [Crossref]
16. Çolakoğlu MK,
Özdemir A, Kalcan S, Demir A, Demiral G et al. (2020) Spontaneous abdomen and
abdominal wall hematomas due to anticoagulant/antiplatelet use: Surgeons'
perspective in a single center. Ulus Travma Acil Cerrahi Derg 26: 50-54.
[Crossref]
17. Vaz de Melo
Trindade G, Pereira TA, Caporali JFM, Vaz de Melo Trindade D, Roriz SJ et al.
(2021) EDTA-dependent pseudothrombocytopenia in patients with hepatosplenic
schistosomiasis mansoni: a clinical management alert. Trans R Soc Trop Med
Hyg 115: 1168-1173. [Crossref]
18. Medeiros TB,
Domingues AL, Luna CF, Lopes EP (2014) Correlation between platelet count and
both liver fibrosis and spleen diameter in patients with schistosomiasis
mansoni. Arq Gastroenterol 51: 34-38. [Crossref]
19. Camacho Lobato L,
Borges DR (1998) Early liver dysfunction in schistosomiasis. J Hepatol
29: 233-240. [Crossref]
20. Pieri S, Agresti P, Buquicchio GL, Di Giampietro I, Trinci M et al. (2015) Endovascular management of the rectus muscle hematoma. Radiol Med 120: 951-958. [Crossref]
21. Albuquerque TVC, Monsignore LM, de Castro Afonso LH, Elias Junior J, Muglia VF et al. (2020) Transarterial embolization with n-butyl cyanoacrylate for the treatment of abdominal wall hemorrhage. Diagn Interv Radiol 26: 216-222. [Crossref]
