From Neuroinflammation to Neuroprotection: Focus on Potential New Therapeutic Targets in Cognitive Impairment
A B S T R A C T
Neurodegeneration is closely linked to neuroinflammation. It is often associated with oxidative stress and meaningful changes in cell energy metabolism. Neuroinflammation is due to non-neuronal cell activation (microglia, astrocytes, mast cells) activation and proliferation. Also, it is associated with pro-inflammatory substances release, able to modify synaptic plasticity. Microglia and astrocytes activation lead to toxic agent’s release (reactive oxygen species, inflammatory cytokines); however, the final target of this process is the cholinergic neuron. A number of substances can promote neuroprotection; recent scientific evidence focuses on the role of sirtuins. In particular, SIRT1 is activated by caloric restriction, NAD biosynthesis and different activators, called STACs (Sirtuin Activating Compounds). Citicoline is one of the most powerful STACs. It has been widely shown to possess neuroprotective action, and lots of studies strengthened its possible role.
Keywords
Neuroinflammation, neuroprotection, cholinergic precursors
From Neuronal Damage to Neuroinflammation
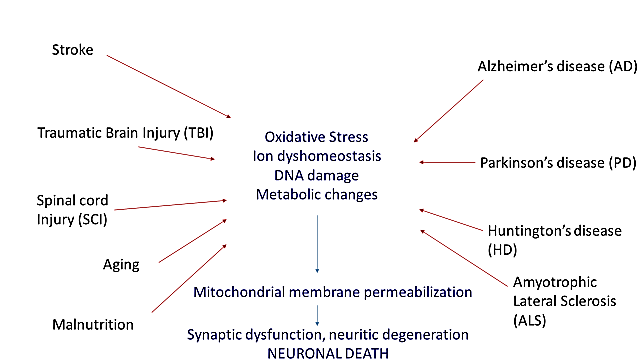
The pathogenetic mechanisms leading to neuronal damage in acute disorders such as stroke, traumatic brain and spinal cord injuries, and neurodegenerative diseases are similar [1, 2]. Pathophysiology of neuronal death involves oxidative stress, metabolic changes, DNA damage, neuronal apoptosis, calcium homeostasis changes and excitotoxicity. The common mechanism leading to neuronal disruption is the loss of mitochondrial membrane permeabilization; furthermore, mitochondrial dysfunction is able to promote neurodegeneration (Figure 1) [1].
However, neuroinflammation is the universally recognized primum movens in neurodegeneration as well as in cognitive impairment [3]. It occurs together with oxidative stress and significant cell energy metabolism. Neuroinflammation is defined as the reactive response of Central Nervous System (CNS) against elements that interfere with homeostasis, inside or outside the CNS, and this response is involved in all neurological diseases, including developmental, traumatic, ischemic, metabolic, infectious, toxic, neoplastic, and neurodegenerative diseases [4]. Astrocytes play a key role in neuron metabolism as well as the so-called dynamic morphofunctional central neuron/non-neuronal cells unit [5]. As a matter of fact, neuroinflammation is linked to the activation and proliferation of non-neuronal cells (precisely astrocytes, microglia, mast cells) and is associated with pro-inflammatory mediator’s release, which in turn are able to change synaptic plasticity.
In other words, the activation of microglia and astrocytes leads to toxic mediator release, such as reactive oxygen species (ROS), inflammatory cytokines; however, the final target is still represented by the cholinergic neuron. Moreover, microglia are cells that act as the first form of immune defense in the brain; while microglia help clear beta-amyloid in the brain, they may become overactive in the presence of beta-amyloid and produce compounds that damage nearby cells [5]. Microglia in the CNS is heterogeneous with different functional phenotypes. Resting microglia are converted to active states of M1 or M2 phenotypes. The M1 phenotype is pro-inflammatory and the M2 phenotype is neuroprotective, immunosuppressive and anti-inflammatory and helps in the tissue healing process [6]. The ratio of M1 and M2 microglial activation is altered in neurodegenerative diseases; in particular, the M2 phenotype promotes the production of anti-inflammatory cytokines IL-4, IL-13, IL-10 e TGF-β which antagonize the activities of pro-inflammatory cytokines to re-establish normal condition [6]. It is also able to promote the expression of cannabinoid receptors CB1 and CB2. The ratio of M1 and M2 microglial activation is altered in neurodegenerative diseases, and therefore, M1/M2 switching may help therapeutic effectiveness in neurodegenerative diseases.
Figure 1: The figure shows how the main mechanisms involving neurodegenerative, vascular, traumatic diseases leading to neuronal damage and death have a common pathogenetic substrate.
For example, Alzheimer’s disease (AD) is a neurodegenerative and multifactorial disease, affecting more than 20 million people worldwide. It is characterized by a progressive deterioration of cognitive functions, particularly memory. An excess in astroglial and microglial activation is typical in AD, which through interacting with abnormal protein aggregates, ultimately leads to dysfunction and neuronal death (neurofibrillary tangles (NFTs), A- and altered tau protein) [7]. Other factors able to promote neuroinflammation can significantly increase the risk of AD, such as traumatic brain injury, high fat intake, B vitamin deficiency, recurrent infections, alterations in cholesterol homeostasis, obesity and poor eating habits [7].
The Role of Cholinergic Precursors in Neuroprotection
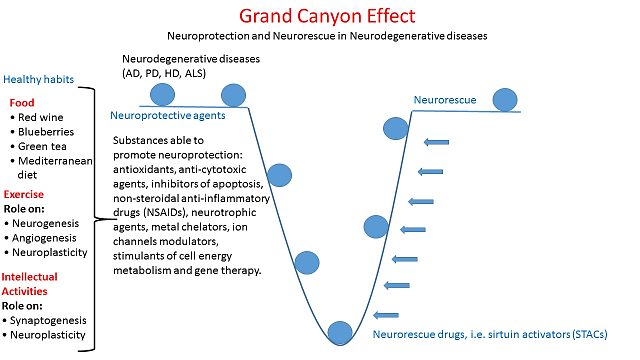
Some healthy habits and a lot of substances are able to promote neuroprotection (Figure 2). In particular, non-steroidal anti-inflammatory drugs (NSAIDs) and COX enzymes can have a crucial role in neuroprotection. COX-inhibiting NSAIDs reduce microglial activation and, on the other hand, neuronal stress processes, such as ischemia and excitotoxicity, are associated with strong upregulation of neuronal COX-2 expression. Moreover, NSAIDs act as peroxisome proliferator-activated receptor- (PPARy agonists) and suppress the expression of a broad range of pro-inflammatory genes [7]. It has also been found that a compound with high concentrations of antioxidants and anti-inflammatory properties, called Andean Compound (fulvic acid), might have strong neuroprotective as well as anti-neuroinflammatory properties [7].
Furthermore, N-palmitoylethanolamide (PEA) has a very similar structure to glutathione, a powerful antioxidant [1, 8]. Luteolin is a flavonoid which exerts its neuroprotective properties through the inhibition of oxidative stress, mast cell degranulation and cytokine release, as well as the autoimmune T-cell activation [1]. In one word, it inhibits microglia activation and proliferation and mimics brain-derived neurotrophic factor (BDNF) [1].
Figure 2: The figure shows some possible neuroprotective habits and substances.
Therefore, all the substances able to inhibit microglial activation and neuroinflammation have marked neuroprotective properties, including resveratrol, curcumin and riluzole [7]. Presently resveratrol is still being studied; it has recognized antioxidant properties and seems to have a protective effect against dopamine-induced cytotoxicity in Parkinson’s disease (PD), as well as in animal models of post-stroke cognitive impairment when being associated to cholinergic precursors such as citicoline [9]. Also, it can attenuate the inflammatory response in activated microglia [7]. Curcumin is a phenolic compound extracted from perennial herb Curcuma longa, characterized for its anti-inflammatory and antioxidant properties. It seems to inhibit microglial proliferation and differentiation, thus reducing the inflammation. Regarding riluzole, it has shown neuroprotective properties in PD and in Amyotrophic Lateral Sclerosis (ALS) by reducing GFAP (glial fibrillary acidic protein) levels in the lesioned striatum in a rodent model [7].
Recent evidence showed sirtuins (SIRTs) to play a crucial role in neuroprotection. SIRTs are NAD-dependent protein deacetylases known to have protective effects against age-related diseases such as cancer, diabetes, cardiovascular and neurodegenerative diseases [10]. SIRT1 is the best-characterized sirtuin in neurons; in particular, SIRT1 activation plays a crucial role in both stroke and AD, whereas SIRT2 seems to be more important in PD [11]. The outstanding protective effects of SIRT1 are due to deacetylation on histone and non-histone targets [11]. SIRT1 is activated by caloric restriction, nicotinamide adenine dinucleotide (NAD) biosynthesis and a number of activators, called STACs (sirtuin activating compounds). One of the most powerful STACs is a cholinergic precursor, CDP-choline (cytidine-5′-diphosphate choline), also called citicoline. Also, as above mentioned, cholinergic neuron represents the common final pathway in neuroinflammatory and neurodegenerative processes.
Citicoline is one of the most frequently pre¬scribed drugs for cognitive impairment all over the world. It is composed of ribose, pyrophosphate, cytosine (a nitrogenous base), and choline, and it has been shown to be effective in cognitive impairment (CI) of diverse etiology, such as in CI following cerebrovascular disease [12]. Citicoline has neuroprotective effects, and it is a physiologically produced endogen compound. It inhibits apoptosis associated with brain ischemia and neurodegeneration; it is able to potentiate neuroplasticity and is a natural precursor of phospholipid synthesis, such as phosphatidylcholine [12]. Citicoline also works as a choline source for acetylcholine biosynthesis in different pathways. Table 1 reports some of its main characteristics.
Table 1: Citicoline’s action [13-16].
|
Increase in acetylcholine intra-synaptic levels |
|
Increase in dopamine and noradrenaline intra-synaptic levels (indirect dopamine-agonist) |
|
Increase in phospholipid synthesis (phosphatidylcholine) |
|
Cell function and neuronal repair stimulation |
|
Inhibition of brain ischemia associated apoptosis |
|
Inhibition of different models of neurodegeneration |
|
Neuroprotection and neuroplasticity boost |
|
Efficacy in the long-term use (6-9 months and beyond) |
Some previous studies (the VITA study, the IDEALE study) have shown the role of citicoline in vascular cognitive impairment [17, 18]. In the wake of other studies which emphasized the role of the cholinergic system in cognitive impairment, the CITIRIVAD study showed that citicoline 1g given orally combined with transdermal rivastigmine, a cholinesterase inhibitor (AchEI), administered at the highest tolerated dosage, slowed down disease progression in patients suffering from AD or mixed dementia assessed after three and nine months from the baseline [19]. Another retrospective and multicentric study, the CITICHOLINAGE study showed that in patients affected with AD, combination treatment of citicoline 1g given orally and AchEIs (rivastigmine, donepezil, galantamine) determined a significant increase in MMSE score (Mini Mental State Examination, a test for assessing cognitive performances), or at least slowed down the cognitive loss in the 9-month observation time. Treatment was safe and well-tolerated, so that it is recommended [20]. Other possible perspectives in the near future could derive from the study of add-on treatment of citicoline with memantine alone or memantine plus AchEIs [21].
It would also be interesting to point out the possible role of citicoline on some outcomes such as the ability to move around, activities of daily living (ADL), mood and sleep, and behavioral disorders. This becomes very important in light of the poor results of various drugs being tested and to try to maximize the current treatments available.
Conflicts of Interest
There are no conflicts of interest to declare. We received no support from industry or organizations which may have influenced this work, and there were no study sponsors involved.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Article Info
Article Type
Review ArticlePublication history
Received: Sat 02, May 2020Accepted: Fri 22, May 2020
Published: Sat 30, May 2020
Copyright
© 2023 Pietro Gareri. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.02.10
Author Info
Antonino Maria Cotroneo Pietro Gareri Valeria Graziella Laura Manfredi
Corresponding Author
Pietro GareriCenter for Cognitive Disorders and Dementia, Catanzaro Lido, ASP Catanzaro, Catanzaro, Italy
Figures & Tables
Table 1: Citicoline’s action [13-16].
|
Increase in acetylcholine intra-synaptic levels |
|
Increase in dopamine and noradrenaline intra-synaptic levels (indirect dopamine-agonist) |
|
Increase in phospholipid synthesis (phosphatidylcholine) |
|
Cell function and neuronal repair stimulation |
|
Inhibition of brain ischemia associated apoptosis |
|
Inhibition of different models of neurodegeneration |
|
Neuroprotection and neuroplasticity boost |
|
Efficacy in the long-term use (6-9 months and beyond) |
References
- Rasool M, Malik A, Qureshi MS, Manan A, Pushparaj PN et al. (2014) Recent Updates in the Treatment of Neurodegenerative Disorders Using Natural Compounds. Evid Based Complement Alternat Med 2014: 979730. [Crossref]
- Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA (2017) Oxidative Stress, Protein Modification and Alzheimer disease. Brain Res Bull 133: 88-96. [Crossref]
- Carriba P, Comella JX (2015) Neurodegeneration and Neuroinflammation: Two Processes, One Target. Neural Regen Res 10: 1581-1583. [Crossref]
- Moskowitz MA, Lo EH, Iadecola C (2010) The Science of Stroke: Mechanisms in Search of Treatments. Neuron 67: 181-198. [Crossref]
- Navarro G, Morales P, Rodríguez Cueto C, Fernández Ruiz J, Jagerovic N et al. (2016) Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front Neurosci 10: 406. [Crossref]
- Gelders G, Baekelandt V, Van der Perren A (2018) Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J Immunol Res 2018: 4784268. [Crossref]
- Morales I, Farías GA, Cortes N, Maccioni RB (2016) Neuroinflammation and Neurodegeneration.
- Beggiato S, Tomasini MC, Ferraro L (2019) Palmitoylethanolamide (PEA) as a Potential Therapeutic Agent in Alzheimer’s Disease. Front Pharmacol 10: 821. [Crossref]
- Hurtado O, Hernández Jiménez M, Zarruk JG, Cuartero MI, Ballesteros I et al. (2013) Citicoline (CDP-choline) Increases Sirtuin1 Expression Concomitant to Neuroprotection in Experimental Stroke. J Neurochem 126: 819-826. [Crossref]
- Donmez G (2013) Sirtuins as Possible Targets in Neurodegenerative Diseases. Curr Drug Targets 14: 644-647. [Crossref]
- Zhang F, Wang S, Gan L, Vosler PS, Gao Y et al. (2011) Protective Effects and Mechanisms of Sirtuins in the Nervous System. Prog Neurobiol 95: 373-395. [Crossref]
- Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G et al. (2015) The Role of Citicoline in Cognitive Impairment: Pharmacological Characteristics, Possible Advantages, and Doubts for an Old Drug With New Perspectives. Clin Interv Aging 10: 1421-1429. [Crossref]
- Secades JJ, Frontera G (1995) CDP-choline: Pharmacological and Clinical Review. Methods Find Exp Clin Pharmacol 17: 1-54. [Crossref]
- Secades JJ (2019) Citicoline in the treatment of cognitive impairment. J Neurol Exp Neurosci 5: 14-26.
- Fioravanti M, Yanagi M (2005) Cytidinediphosphocholine (CDP-choline) for Cognitive and Behavioural Disturbances Associated With Chronic Cerebral Disorders in the Elderly. Cochrane Database Syst Rev 18: CD000269. [Crossref]
- Hurtado O, Lizasoain I, Moro MÁ (2011) Neuroprotection and Recovery: Recent Data at the Bench on Citicoline. Stroke 42: S33-S35. [Crossref]
- Putignano S, Gareri P, Castagna A, Cerqua G, Cervera P et al. (2012) Retrospective and Observational Study to Assess the Efficacy of Citicoline in Elderly Patients Suffering From Stupor Related to Complex Geriatric Syndrome. Clin Interv Aging 7: 113-118. [Crossref]
- Cotroneo AM, Castagna A, Putignano S, Lacava R, Fantò F et al. (2013) Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clinical Interv Aging 8: 131-137. [Crossref]
- Castagna A, Cotroneo AM, Ruotolo G, Gareri P (2016) The CITIRIVAD Study: CITIcoline plus RIVAstigmine in Elderly Patients Affected with Dementia Study. Clin Drug Investig 36: 1059-1065. [Crossref]
- Gareri P, Castagna A, Cotroneo AM, Conforti R, Santamaria F et al. (2017) The Citicholinage Study: Citicoline Plus Cholinesterase Inhibitors in Aged Patients Affected with Alzheimer's Disease Study. J Alzheimer’s Dis 56: 557-565. [Crossref]
- Gareri P, Cotroneo AM, Orsitto G, Putignano S (2020) The CitiMem study: optimizing pharmacological treatment in dementia. Arch Geront Geriatr 89: 104073.
