Endometriosis in a Renal Transplant Recipient
A B S T R A C T
Hormonal abnormalities that are associated with advanced kidney failure normally reverses after kidney transplantation. This usually helps to normalize menstrual cycles for female patients and helps improve fertility. Post-transplant gynaecological disorders are under-reported in general. We present a patient who developed endometriosis after second kidney transplant. She was treated with surgery followed by hormonal therapy. We discuss the pathophysiology of endometriosis and possible relation to the immune system.
Keywords
Kidney transplantation, endometriosis, immunosuppression
Background
Organ transplantation is now a universally accepted treatment for organ failure. Kidney Transplantation improves end-stage renal disease patients’ survival and quality of life in comparison with dialysis. Hypothalamic pituitary axis normalizes post-transplant. Some of the well-known gynaecological complications post-transplant are prolonged and profuse menstruation and inter-menstrual bleeding or spotting. Reports also suggest a higher rate of endometrial hyperplasia (without atypia) in renal allograft recipients [1, 2]. After successful renal transplantation, cyclical ovarian function and fertility are often restored.
Case History
We present a case of a 33-year-old unmarried female who underwent a second living unrelated kidney transplant In September 2017 with immediate graft function and uneventful post-operative recovery. Her first transplant was a combined deceased donor kidney and heart transplant that was done in December 2014 and complicated with loss of renal allograft from antibody-mediated rejection leading to return to dialysis in May 2017 while the heart transplant continued to function well. Her original heart disease was idiopathic Myocarditis while her original kidney disease was thought to be focal segmental glomerulosclerosis (FSGS) that got worse with worsening heart failure.
She was treated aggressively for the antibody-mediated rejection, but despite that allograft dysfunction and proteinuria continued to progress. Following her second kidney transplant, she was placed on a triple immunosuppressive regimen of Tacrolimus, Mycophenolate mofetil, and Prednisolone. Serum creatinine settled at 60-80 micromole/L and proteinuria disappeared.
In February 2018 she presented to the clinic with right lower quadrant pain started in December 2017, waxing, and waning in character and gradually worsening to the point where she was unable to sit, stand, or sleep without having pelvic fullness and pain as well as bloating. Her menstrual cycles were regular till early January 2018 when she started to have bleeding twice a month.
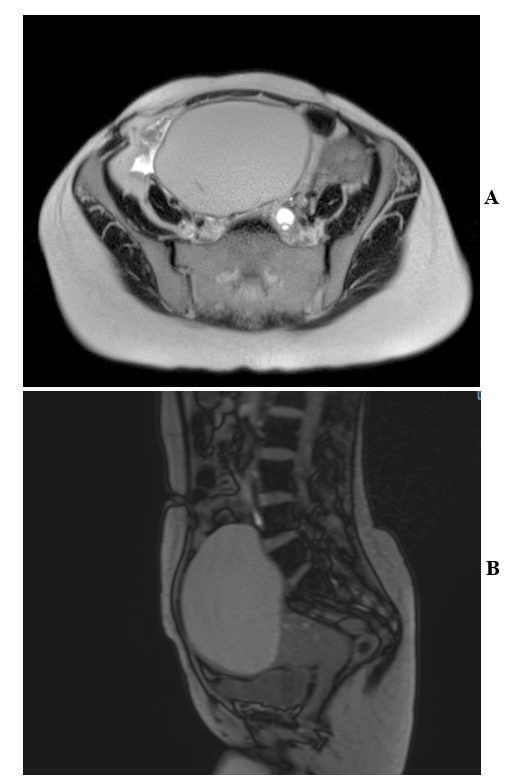
On further questioning, she admitted to having always had painful periods. No family members with known endometriosis. She denied any family history of ovarian, colorectal or breast malignancy. MRI scan was done and revealed a right 13 cm complex, septated, hemorrhagic debris-filled ovarian cyst (Figures 1A & 1B). The patient was taken up for surgical removal of the cyst and argon laser coagulation of endometrial implants. Intra-operative findings revealed grade 3 endometriosis involving bilateral fallopian tubes and uterus had dense posterior adhesions to the rectum and right pelvic sidewall and had anterior adhesions to bladder highly suggestive of endometriosis. The patient has normal postoperative renal recovery with no evidence of acute kidney injury. Post-surgery, the patient was maintained on Medoxy-progesterone acetate every 3 months. She is currently asymptomatic with stable kidney function and no proteinuria.
Figures 1: MRI pelvis with contrast in A) axial view and B) sagittal view showing large midline pelvis mass reported to be arising from the right ovary containing multiple septations which does not enhance clearly. It contains T2 dark and T1 bright non-enhancing debris. The mass is diffusely T1 bright, contains dependent debris which is more T1 bright than the remainder of the mass.
Discussion
Endometriosis is an estrogen-dependent inflammatory disease, which affects 6%–10% of women of reproductive age [3-5]. This disease is defined as the presence of endometrial glandular and stromal tissues in sites outside the uterine cavity, primarily on ovaries and the pelvic peritoneum [6]. The Association of CKD with endometriosis is not well studied. A few case reports of obstructive uropathy showed an association with endometriosis [7-10]. A retrospective analysis on 27,973 patients revealed endometriosis to be inversely proportional to CKD (crude HR 0.65, 95% CI 0.53–0.81, p < 0.001) [11]. However, in another similar study designed to determine the prevalence of organic causes of abnormal uterine bleeding in this group of patients exposed to unopposed estrogen, only 2 of 8 patients (25%) with chronic renal failure had endometrial lesions while 44 of 131 patients (33.6%) had either endometrial polyp, simple or atypical endometrial hyperplasia or endometrial carcinoma (p > 0.05) [12].
Patients with secretory and atrophic endometrium were excluded from this study. Uremia in CKD may have no role to play in endometrial responsiveness to estrogen. Despite the substantial effect that endometriosis has on women, their families and the economy, public and professional awareness of the disorder remains poor [13]. The true prevalence of endometriosis is uncertain, however, because the definitive diagnosis requires surgical visualization. The prevalence ranges from 2 to 11% among asymptomatic women, 5 to 50% among infertile women, and 5 to 21% among women hospitalized for pelvic pain.
Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. The dysfunction of the innate and adaptive immune system is evident but it is unclear whether immune dysfunction initiates endometriosis or is a pathophysiological hallmark of the disorder [14]. However, in general, it seems there is a local and sterile inflammation that occurs in the peritoneal cavity and there is substantial evidence that immunological factors and angiogenesis play a decisive role in the pathogenesis of the disease. Peritoneal macrophages also play a pivotal role in the intraabdominal environment [15]. Theoretically, immunosuppressive therapy would halt those processes and that is why probably it is uncommon to see patients with endometriosis after transplantation. Other mechanisms contribute to the development of endometriosis. That is probably why it happened in this immunocompromised patient. On the other hand, in our literature search, we found only animal studies done on the effects of immunosuppression on development and progression of endometriosis and this was done on baboons and the result was largely inconclusive [16, 17].
Conclusion
In this case report, we bring forward the importance of gynaecological complications following renal transplant which arises due to normalizing hormonal balance after transplant. Gynaecological complications following transplant are under-reported. Our case report is unique in this aspect as this is the first case of post-transplant endometriosis ever to be reported thus far. Theoretically, the prevalence of endometriosis must have been mitigated in a transplant patient however this case report shows that more studies need to be done in this front.
Consent
Informed consent was obtained from the patient prior to writing this report.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Thu 23, Apr 2020Accepted: Wed 06, May 2020
Published: Wed 13, May 2020
Copyright
© 2023 Nizar Attallah. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.TCR.2020.01.07
Author Info
Ammar Abdulbaki Nizar Attallah Rakesh Madhyastha Sudeendra Gupta
Corresponding Author
Nizar AttallahDepartment of Nephrology, Medical Subspecialties Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi, UAE
Figures & Tables
References
- Kaminski P, Bobrowska K, Pietrzak B, Bablok L, Wielgos M (2008) Gynecological issues after organ transplantation. Neuro Endocrinol Lett 29: 852-856. [Crossref]
- Ruth Cochrane, Lesley Regan (1997) Undetected gynaecological disorders in women with renal disease. Human Reproduction 12: 667-670.
- Lee WL, Chang WH, Wang KC, Guo CY, Chou YJ et al. (2015) The risk of epithelial ovarian cancer of women with endometriosis may be varied greatly if diagnostic criteria are different: A nationwide population-based cohort study. Medicine (Baltimore) 94: e1633. [Crossref]
- Chang WH, Wang KC, Lee WL, Huang N, Chou YJ et al. (2014) Endometriosis and the subsequent risk of epithelial ovarian cancer. Taiwan J Obstet Gynecol 53: 530-535. [Crossref]
- Wang KC, Chang WH, Lee WL, Huang N, Huang HY (2014) An increased risk of epithelial ovarian cancer in Taiwanese women with a new surgico-pathological diagnosis of endometriosis. BMC Cancer 14: 831. [Crossref]
- Bulun SE (2009) Endometriosis. N Engl J Med 360: 268-279. [Crossref]
- Mourin-Jouret A, Squifflet JP, Cosyns JP, Pirson Y, Alexandre GP (1987) Bilateral ureteral endometriosis with end-stage renal failure. Urology 29: 302-306. [Crossref]
- Hsieh MF, Wu IW, Tsai CJ, Huang SS, Chang LC et al. (2010) Ureteral endometriosis with obstructive uropathy. Intern Med 49: 573-576. [Crossref]
- Muñoz JL, Jiménez JS, Tejerizo A, Lopez G, Duarte J (2012) Rectosigmoid deep infiltrating endometriosis and ureteral involvement with loss of renal function. Eur J Obstet Gynecol Reprod Biol 162: 121-124. [Crossref]
- Arrieta Bretón S, López Carrasco A, Hernández Gutiérrez A, Rodríguez González R, de Santiago García J (2013) Complete loss of unilateral renal function secondary to endometriosis: A report of three cases. Eur J Obstet Gynecol Reprod Biol 171: 132-137. [Crossref]
- Huang BS, Chang WH, Wang KC, Huang N, Guo CY (2016) Endometriosis Might Be Inversely Associated with Developing Chronic Kidney Disease: A Population-Based Cohort Study in Taiwan. Int J Mol Sci 17: E1079. [Crossref]
- Ergeneli MH, Duran EH, Zeyneloglu HB, Demirhan B, Erdogan M (1999) Endometrial response to unopposed estrogens remains unaltered in patients with chronic renal failure receiving hemodialysis. Gynecol Obstet Invest 47: 26-28. [Crossref]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A et al. (2012) The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 27: 1292-1299. [Crossref]
- Zondervan KT, Becker CM, Missmer SA (2020) Endometriosis. N Engl J Med 382: 1244-1256. [Crossref]
- Gazvani R, Templeton A (2002) Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 123: 217-226. [Crossref]
- D Hooghe TM (1997) Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril 68: 613-625 [Crossref]
- D Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Hill JA et al. (1995) The effects of immunosuppression on development and progression of endometriosis in baboons (Papio anubis). Fertil Steril 64: 172-178. [Crossref]
