En Bloc Capsulectomy with Implant Explantation in a High-Risk Patient with Breast Implant Illness: A Case Report
A B S T R A C T
En bloc resection with capsulectomy in breast implant surgery may be problematic and challenging, with limited indications aside from Breast Implant Associated Anaplastic Large Cell Lymphoma [1, 2]. This is a case report highlighting the technique and tools utilized intraoperatively in higher risk circumstances with a 35-year-old female patient and her medical team requesting explantation with total capsulectomy as a compassionate measure for a perceived contribution towards the patient’s uncontrolled hypertension of unknown etiology [3].
Keywords
Capsulectomy, en bloc, explantation, breast implant illness
Introduction
The role of silicone breast implants in various medical conditions is ever evolving. The most worrisome condition is Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) [4]. The treatment for this is complete capsulectomy with implant explantation [1, 2]. En bloc removal of the capsule can be problematic and challenging in any patient even under ideal elective circumstances. We present our experience with a case performed under higher risk circumstances.
Methods
Patient AB was a 35-year-old woman admitted to the medical ICU for treatment of uncontrolled hypertension. The patient was transferred from an outside facility with co-morbidities of cerebrovascular accident – then on anticoagulation, and aspiration pneumonia – receiving antibiotics with a resultant tracheostomy. Her hypertension remained uncontrolled while on three intravenous antihypertensive medications.
The patient had a very extensive workup, including consultation with nephrology, cardiology, infectious disease, allergy, as well as multisystem imaging and extensive laboratory testing. No source or etiology for her uncontrolled hypertension was identified. The patient's family and the managing service requested consultation from plastic surgery to evaluate for the possibility that saline breast implants may be the etiology of her condition. The patient had saline implants placed submuscular via periareolar incision five years prior. This was done elsewhere, and no records were available. As per history, there were no problems related to implant placement and healing and no complaints specific to the implants. Hypertension developed 3 years after placement of the implants. The patient failed at outpatient management of hypertension, with many sequala including renal failure, vision changes and subsequent stroke requiring hospital admission.
Physical examination revealed the patient’s BMI as 19. She had minimal muscle and breast tissue overlying the implants. Incisions were well healed and grade 1 capsule bilateral. There was no asymmetry, skin changes, induration or axillary adenopathy.
Extensive discussion with the family and the managing service was conducted. Based on our review of the literature and discussion with colleagues locally and nationally, saline breast implants were not the likely cause of her symptoms and pathology [3, 5]. The managing service stated clearly to us that they did not have a definitive treatment plan for the patient, and all workup was negative. They were reasonably certain that without some correction of her hypertension, she would most likely suffer demise from some complication of hypertension. We were asked to consider en bloc resection as a compassionate intervention. We explained that from a technical standpoint, complete radical capsulectomy would risk breast skin loss, inadvertent thoracotomy, postoperative bleeding from anticoagulation and risk of mortality from a prolonged surgery. After extensive discussion, informed consent was obtained.
Procedure
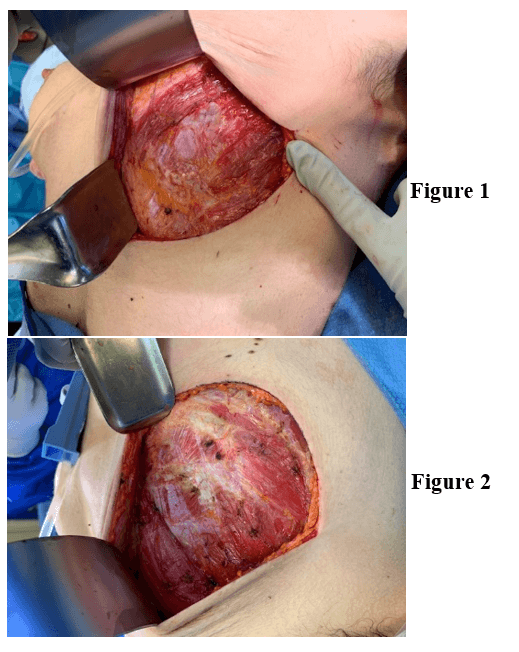
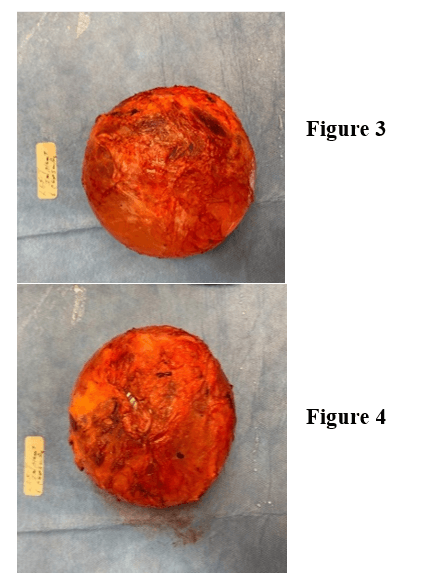
Under general anesthesia, an inframammary incision was made 10 cm in length, slightly shorter than the palpable diameter of the implant. This was taken down to the deep subcutaneous tissue (Figures 1 & 2). A Medtronic plasmablade electrocautery was used to minimize bleeding and improve visualization. With manual downward pressure on the implant, a small layer of fat was left on capsule and dissection was carried over the top of the capsule with a retractor providing superior and anterior traction. With palpation of the implant, we were able to identify the subcutaneous layer just off the implant. Dissection was continued superiorly and bilaterally for approximately 50% of the implant. The retractor was then placed below the implant capsule to delineate the plane above the ribs. Electrocautery dissection was used to expose the periosteum and then advanced to the next rib. This was continued until the inferior capsule plane was exposed. We were then able to deliver the implant and capsule intact through the incision. The coagulation setting 8 was used on the Medtronic Plasmablade to minimize bleeding. The implant was delivered with intact capsule, bilateral (Figures 3-6). The wound was explored and irrigated. The incisions were closed over suction drains. There were no complications in the immediate postoperative period. No evidence of bleeding was noted through the drains or change in lab values. The drains were removed at two weeks. At three weeks post operation, the right breast was noted to have redness and induration. An ultrasound confirmed a complex fluid collection. This was drained at bedside, and the patient was given a course of oral antibiotics. The patient was discharged from the institution on multiple antihypertensive medications. The discharge was three weeks after completion of the procedure.
Figures 1 & 2: Surgical incision and exposure.
Figures 3 & 4: Left implant with capsule.
Figures 5 & 6: Right implant with capsule.
Discussion
There is increasing demand on plastic surgeons to remove breast implants with intact capsule. Other than BIA-ALCL, the rationale for this may not be clear. It is, therefore, important to minimize risk and morbidity. Unlike simple explantation, en bloc resection requires erring on removing normal tissue to avoid entering the implant capsule. Specialty technology, such as radiofrequency energy electrosurgery, controls bleeding precisely while also limiting thermal damage to the surrounding tissue. This technology is well suited for this type of case, especially to limit surgery time and minimize the risk of complications. We encourage others to weigh the risk and benefits of complete en bloc explant capsulotomy while determining the best technique for each patient.
Funding
None.
Abbreviations
BIA-ALCL: Breast Implant Associated Anaplastic Large Cell Lymphoma
Article Info
Article Type
Case ReportPublication history
Received: Tue 07, Apr 2020Accepted: Wed 22, Apr 2020
Published: Wed 29, Apr 2020
Copyright
© 2023 Zuhair Irfan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.04.06
Author Info
Aamir Siddiqui Donna Tepper Zuhair Irfan
Corresponding Author
Zuhair IrfanDepartment of Plastic Surgery, Henry Ford Hospital, Detroit, Michigan, USA
Figures & Tables

References
- Clemens MW, Jacobsen ED, Horwitz SM (2019) 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 39: S3-S13 [Crossref]
- Swanson E (2020) Evaluating the Necessity of Capsulectomy in Cases of Textured Breast Implant Replacement. Ann Plast Surg. [Crossref]
- Adidharma W, Latack KR, Colohan SM, Morrison SD, Cederna PS (2020) Breast Implant Illness: Are Social Media and the Internet Worrying Patients Sick? Plast Reconstr Surg 145: 225e-227e. [Crossref]
- Dashevsky BZ, Gallagher KM, Grabenstetter A, Cordeiro PG, Dogan A et al. (2019) Breast implant‐associated anaplastic large cell lymphoma: Clinical and imaging findings at a large US cancer center. Breast J 25: 69-74. [Crossref]
- FDA Update on the Safety of Silicone Gel-Filled Breast Implants (2011) Center for Devices and Radiological Health U.S. Food and Drug Administration.
- Singh N, Picha GJ, Hardas B, Schumacher A, Murphy DK (2017) Five-year safety data for more than 55,000 subjects following breast implantation: comparison of rare adverse event rates with silicone implants versus national norms and saline implants. Plast Reconstr Surg 140: 666-679. [Crossref]
