Effects of DSPP and MMP20 Silencing on Key Signaling Pathways in Oral Squamous Cell Carcinoma Cells
A B S T R A C T
Introduction: Oral carcinogenesis is a multistage process, featuring genetic and molecular alterations leading to rapid cell division, invasion, metastasis, and increased cell survival. Many of these alterations are due to perturbations in the cell signaling networks, which in turn lead to constitutive deregulation of the proteins involved in the regulatory pathways. Our recent reports show that the silencing of dentin sialophosphoprotein (DSPP) and its cognate matrix metalloproteinases 20 (MMP20) alters key tumorigenic hallmarks of oral squamous cell carcinoma (OSCC).
Objective: This study, intended to advance our recent findings, focuses on determining the effects of silencing DSPP and its cognate MMP20 on the signaling pathways that control cell proliferation, differentiation, invasion and metastasis.
Materials and Methods: DSPP and MMP20 were silenced individually and in combination, using adenovirus-mediated short hairpin RNA (shRNA) in OSCC cell line, OSC2, and the effects of silencing on the following pathways: EFGR; RAS-RAF; MEK; MAPK; ERK; JNK; NF-kB; TGFβ; and GSK3β, were analysed by western blot.
Results: DSPP and MMP20 silencing decreased EGFR, KRAS, MEK1/2, MAPK, ERK, MEEK1, JNK, CREBP, p300, NF-kB,TGF β, SMAD7, GSK3 β, and β-catenin expressions. In contrast, the expression of IKKα and SMAD4 were increased in DSPP/MMP20-silenced group, compared with control group. Furthermore, DSPP-silencing alone was more effective than MMP20, or combined DSPP-MM20 silencing, in altering the levels of key proteins of each signaling pathway investigated.
Conclusion: Our findings provide the basis for further studies aimed at verifying the effects of these alterations in the profiles of these proteins on the various hallmarks of oral carcinogenesis, and for understanding the molecular role of DSPP and MMP20 in OSCC. This is with a view to evaluating their diagnostic and prognostic utility as well as the values of DSPP/MMP20 as potential targets for design of chemotherapeutic agents for the treatment of OSCC patients.
Keywords
DSPP, MMP20, signaling in oral cancer, EFGR, RAS/RAF, MAPK, JNK, CREBP, p300, NF-kB, IKKα, TGF β, SMAD, GSK3β, β catenin
Introduction
Oral squamous cell carcinoma (OSCC), accounting for over 90% of head and neck squamous cell carcinomas (HNSCCs), may arise from any region of the oral mucosa [1]. OSCC is characterized by tumor aggressiveness and high recurrence rate following surgical treatment of primary disease [2]. The main etiologic agents remain smoking and other forms of tobacco use, alcohol and, in recent years, human papillomavirus (HPV) infection [3]. OSCCs often are preceded by premalignant precursors in the forms of leukoplakia and erythroplakia [4]. The multistep progression of oral premalignant lesion (OPL) to invasive OSCC involves activation of the tumor progression and the inhibition of tumor suppressor pathways [5]. However, unlike epithelial cancers of other regions, our understanding of the molecular mechanism underlying the progression of OSCC is currently lagging. Notable dysregulated pathways in OSCCs, include EGFR, RAS/RAF, Wnt/β-catenin, TGF- β, and PI3K-AKT-mTO [6]. It is therefore conceivable that blocking upstream signaling in these pathways may impede the development of OSCC.
We and others have reported the potential role of dentin sialoprotein phosphoprotein (DSPP), a member of the small integrin binding ligand N-linked glycoprotein (SIBLING) and its cognate matrix metalloproteinase partner, MMP20, in various cancers [7-12]. Specifically, upregulation of DSPP in dysplastic OPLs and in OSCCs correlated with tumor aggressiveness [12]. Recently we reported that silencing DSPP and MMP20 in OSCC cell line downregulated proteins known to mediate cell adhesion, metastasis, angiogenesis, epithelial mesenchymal transition (EMT), and cancer stemness [13].
As a next logical step towards our complete understanding of the roles of DSPP and MMP20 in the overall biology of OSCC, we investigated the effects of DSPP and MMP20 silencing in OSCC cell line on the various signaling pathways implicated in OSCC. The effects of DSPP-MMP20 silencing on proteins involved in the EFGR, RAS-RAF, MEK, MAPK, ERK, JNK, NF-kB, TGFβ and GSK3β pathways were analysed by western blot. The results of the study show that silencing DSPP and MMP20 downregulated proteins involved in the downstream signaling in EGFR, RAS-RAF, MAPK, NF-kB, TGFβ GSK3β and β catenin pathways. The results also showed that the levels of inhibitory proteins: IKKα; SMAD4; and DKK1 in these pathways were significantly upregulated in the groups silenced for DSPP and MMP20.
Materials and Methods
Appropriate Institutional Review Board approval for this study was obtained from the University of Texas Health Science Center at Houston.
I Cell Line and Culture Conditions
Previously validated OSCC cell line, OSC2, initially obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) as a model cell line for investigating SIBLING/MMP interaction in oral cancer was used for this study [14]. Also, our recently established and published stable lentiviral-mediated DSPP-, MMP20-, combined DSPP-MMP20-silenced OSC2 phenotypes, and scrambled (ShC) controls were similarly validated [14]. ShRNA plasmid A, used as negative control, was obtained from Santa Cruz Biotechnology (cat. #sc-108060; Santa Cruz, CA, USA). We routinely validate these stably silenced phenotypes of OSC2 cell lines (75% silencing) and did so prior to use in this present study. The stably silenced phenotypes were cultured as a monolayer in DMEM/F12 medium containing 10% FBS (Invitrogen, Carlsbad, CA, USA), supplemented with 1% penicillin/streptomycin and 500 ng/mL hydrocortisone (Sigma Aldrich, St. Louis, MO, USA), and maintained in the presence of 5% CO2 humidified air at 37 °C.
II Western Blot (WB)
Total cell lysates were prepared from each group (control, shDSPP, shMMP-20, and shDM) and 50µg of protein was resolved on SDS-PAGE gel using the mini-protean tetra cell unit (Biorad, Hercules, CA, USA), and transferred to a polyvinylidene (PVDF) membrane (Millipore; Burlington, MA, USA). The membrane was blocked with 5% milk for an hour before incubating in primary antibodies. Primary antibodies for EGFR (sc71033), p300 (sc584), NF-kB p50 (sc 53744), NF-kB p65 (sc 8008) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), whereas antibodies to KRAS (PA5-44339), MEK1/ MEK 2 (PA1-10034), p38 MAPK (LF-MA0126), ERK1/ERK2 (13-6200), MEEK1 (PA5- 43209), CREBP (PA1-847), IKKα (lk ma0161), TGFβ (F.888.7), GSK3β (399500) and DKK1 (38-1200) were purchased from Invitrogen (Carlsbad, CA). Primary antibodies to JNK (51151-1-AP), SMAD4 (MA5-15682), SMAD (42-0400), and β catenin (ma1-3000) were purchased from Thermo Fisher (Carlsbad, CA), whereas, antibody to the house-keeping gene β actin (ab4970) was purchased from Abcam (San Francisco, CA). Following overnight incubation with primary antibody, the membrane was washed 3X with TBST, incubated in secondary antibodies, and signal detected using infrared LI-COR imaging system (LI-COR Biosciences; Lincoln, NE, USA). Quantification of the proteins was performed using actin as the internal control. Protein expression is quantified as fold difference relative to the control. All experiments were performed in triplicates and data were expressed as mean ± SD.
III Statistical Analysis
Statistical analysis was performed using SigmaStat version 3 (Systat software, Point Richmond, CA, USA) and SAS 9.1 (SAS Institute, Inc., Cary, NC, USA). The Kruskal-Wallis test and Dunn multiple comparisons were used any time more than two groups were compared. The criteria for significance were p < 0.05, p < 0.01, and p < 0.001 for this study.
Results
Our previous report shows that silencing DSPP and MMP20 in OSCC cell line (OSC2) altered various tumorigenic hallmarks of OSCC [13]. Our next step was to elucidate the signaling pathways implicated in these alterations following DSPP-MMP20 silencing. To this end, EGFR, MAPK, RAS/RAF, NFkB, TGFβ and β catenin pathways were analysed by western blot following DSPP and MMP20 silencing OSCC cell lines, OSC2.
I EGFR is Downregulated Following DSPP/MMP20 Silencing
EGFR plays an important role in regulation of cell proliferation, survival and differentiation, is aberrantly activated in tumors and associated with tumor progression [15]. As shown in (Figure 1A), EGFR levels were significantly reduced in OSC2 cells following DSPP and MMP20 silencing. In DSPP silenced cells, EGFR decreased by 96% (p<0.001), by 46% (p<0.05) in MMP20 silenced cells, and by 67% in combined DSPP-MMP20 silenced cells (p<0.01). The greater singular effect of DSPP silencing, compared with MMP20 and combined DSPP-MMP20 silencing, indicates that the effect of DSPP and MMP20 silencing is not synergistic, and DSPP may exert more oncogenic role than MMP20 in OSCC.
Figure 1: Westen blots of EGFR, KRAS, MEK 1/2, P38 MAPK and ERK ½ in OSCC cells.
A) The protien expression of EGFR decreased by 96% in shDSPP cells, 46% in shMMP20 cells (p<0.05), and 67% in shDM cells (p< 0.01), compared with control. B) KRAS decreased significantly by 52%, 59 and 49% in shDSPP, shMMP20 and shDM cells, respectively (p<0.001), compared with shC. C) The expression of MEK1/MEK2 decreased by 33%, 34% and 30% in shDSPP, shMMP20 and shDM cells, respectively (p<0.05 and p<0.01), compared with shC. D) MAPK levels decreased by 37% in shDSPP (p<0.05), 48% in shMMP20 (p<0.01) and 62% in shDM cells (p<0.01) compared with shC. E) ERK1/ERK2 levels decreased by 76% in shDSPP, 73% in shMMP20, and by 64% in shDM (p< 0.001) compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.
II MEEK1, JNK, CREBP, p300 of Ras/Raf Signaling are Downregulated Following DSPP/MMP20 Silencing
The Ras signaling pathway is deregulated in oral cancers. As shown in (Figure 1B), KRAS is significantly decreased by 52%, 59%, 49% following DSPP, MMP20, and combined DSPP-MMP20 silencing, respectively (p<0.001), with the effects of individual silencing more profound than the effect of DSPP-MMP20. The expression of MEK1/MEK2 decreased by 33%, 34% and 30% in DSPP, MMP20 and combined DSPP-MMP20 silenced cells, respectively (Figure 1C; p<0.05; p<0.01), whereas the MAPK levels (Figure 1D) decreased by 37%, 48%, and 62% in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively (p<0.01), compared with ShC control. Notably, decreased levels of MAPK was much more in combined DSPP-MMP20 silenced cells than in DSPP, or MMP20 silenced cells. As shown in (Figure 1E), ERK1/ERK2 levels decreased by 76%, 73%, and 64% in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively (p<0.001) compared with shC control. MEEK1 and JNK receive signals from the RAS pathway. Western blot showed MEEK1 level decreased by 72%, 50%, and 48% in DSPP, MMP20, and DSPP-MMP20 silenced cells, respectively (p<0.001), compared with shC control (Figure 2a). This suggests that the effects of DSPP silencing on MEEK1 was more profound compared with MMP20 or combined DSPP-MMP20 silencing. Similarly, JNK levels were decreased by 47%, (p<0.01), 33% (p<0.05), and 42% (p<0.05) in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively, compared with shC control (Figure 2b).
Figure 2: Western blot of MEEK1, JNK, CREBP and p300 in OSCC cells.
a) MEEK1 protein expression decreaed by 72% in shDSPP cells (p<0.001), 50% in shMMP20 cells (p<0.01), and 48% in shDM cells (p< 0.01), compared with ShC. b) JNK decreased significantly by 47% (p<0.01), 33%, (p<0.05), and 42% (p<0.05) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. c) The expression of CREBP decreased by 72% (p<0.01), 76% (p<0.01), and 48% (p<0.05) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. d) p300 levels decreased by 58% in shDSPP (p<0.01), 45% in shMMP20 (p<0.05) and 62% in shDM cells (p<0.01) compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.
III CREBP and p300 Levels are Decreased Following DSPP/MMP20 Silencing
Upregulation of CREBP and p300 are associated with oral cancer progression and decreased patient survival. Western blot showed the protein expression of CREBP decreased by 72% (p<0.01), 76% (p<0.01), and 48% (p<0.05) in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, compared with scrambled controls (Figure 2c). Similarly, p300 levels significantly decreased by 58% (p<0.01), 45% (p<0.05), and 62% (p<0.01) in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively, compared with scrambled controls (Figure 2d).
IV DSPP/MMP20 Silencing Downregulates NF-kB and Upregulates IKKα
Figure 3 shows analysis of western blot of p50 and p65 subunits of NF-kB, and their inhibitor IKKα in DSPP and MMP20 silenced cells. With respect to p50 subunit of NFkB, there was a decrease in 46% (p<0.05), 39% (p<0.05), and 41% (0.01) in the levels of DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively (Figure 3A). The p65 subunit decreased by 74%, 56%, and 55% in DSPP, MMP20, and DSPP-MMP20 silenced cells, respectively (p<0.01), compared with controls. In contrast, IKKα levels increased significantly following DSPP, MMP20, and combined DSPP-MMP20 silencing: 2-fold in DSPP, 1.7-fold in MMP20, and 2.1-fold in combined DSPP-MMP20 silenced cells, compared with controls (Figure 3C).
Figure 3: Western blot of NFkBp50, NFkB p65 and IKKα.
A) Protein expression of NFkB p50 subunit decreased by 46% in shDSPP cells (p<0.05), 39% in shMMP20 cells (p<0.01), and 41% in shDM cells (p< 0.05), compared to control. B) p65 subunit of NFkB decreased significantly by 74% (p<0.05), 56%, (p<0.05), and 55% (p<0.01) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. C) The expression of IKKα increased by 2-fold (p<0.01), 1.7-fold (p<0.01), and 2.1-fold (p<0.01) in shDSPP, shMMP20 and shDM cells respectively, compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.
V TGFβ, SMAD4, and SMAD7 are Downregulated Following DSPP/MMP20 Silencing
The levels of TGFβ, SMAD4, and SMAD7, implicated in the TGFβ signaling pathway, were analysed by western blot following DSPP/MMP20 silencing in OSC2 cells. As shown in (Figure 4), TGFβ level significantly decreased by 55% (p<0.01), 30% (p<0.05), and 53% (p<0.01) in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively, compared with controls (Figure 4A). The expression of SMAD7 decreased by 47% (p<0.01), 74% (p<0.05), and 63% (p<0.05) following DSPP, MMP20, and combined DSPP-MMP20 silencing, respectively, compared to controls (Figure 4B). In contrast, SMAD4 levels increased by 1.5-fold (p<0.01), 1.7-fold (p<0.01), and 2-fold (p<0.001) in DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively, indicating that a combined DSPP-MMP20 silencing was most effective in suppressing SMAD4 levels (Figure 4C).
Figure 4: Western blot of TGFβ, SMAD4 and SMAD7 in OSCC cells.
A) Protein expression of TGFβ decreased by 55% in shDSPP cells (p<0.01), 30% in shMMP20 cells (p<0.05), and 53% in shDM cells (p< 0.01), compared with ShC. B) SMAD7 levels decreased significantly by 47% (p<0.01), 74%, (p<0.05), and 63% (p<0.05) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. C) The levels of SMAD4 increased by 1.5-fold (p<0.01), 1.7-fold (p<0.01), and 2.0-fold (p<0.001) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.
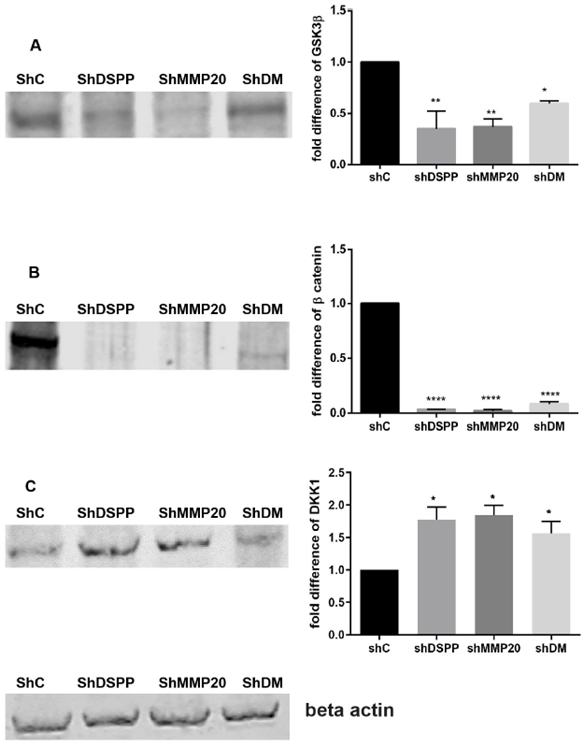
VI GSK3β and β-catenin are Downregulated, whereas Levels of DDK1 is Upregulated Following DSPP/MMP20 Silencing
Published reports indicate that the Wnt/β-catenin signaling pathway is upregulated in several cancers, including oral squamous cell carcinoma [16]. Western blot analysis of three key proteins of the Wnt/β-catenin signaling pathway: GSK3β; β-catenin; and DDK1, showed that levels of GSK3β decreased significantly by 65% (p<0.01), 64% (p<0.01), and 41% (p<0.05) for DSPP, MMP20, and combined DSPP-MMP20 silenced cells, respectively, compared with controls (Figure 5A). The levels of β-catenin were almost undetectable following DSPP (97% reduction; p<0.001), MMP20 (99% reduction; p<0.001), and combined DSPP-MMP20 (92% reduction; p<0.001) silencing (Figure 5B). In contrast, levels of the GSK3β inhibitor, DDK1, was significantly increased 1.7-fold (p<0.05), 1.8-fold (p<0.05), and 1.8-fold (p<0.05) in DSPP, MMP20, and DSPP-MMP20 silenced cells (Figure 5C). This suggests that the primary effects of DSPP/MMP20 silencing on Wnt/β-catenin signaling may be to upregulate DDK1, which in turn suppresses GSK3β and β-catenin expression.
Figure 5: Western Blot of GSK3β, β-catenin, and DKK1 in OSCC cells.
A) Protein expression of GSK3β decreased by 65% and 64% and 41% respectively in the DSPP, MMP20 and DM group (p<0.01) (p<0.05). B) The expression of β catenin was almost nil in all the groups under investigation (97%, 99% and 92% in the DSPP, MMP20 and DM group (p<0.001). C) The expression of DKK1 increased by 1.7-fold in the DSPP silenced group and 1.6- fold in the MMP20 and DM group (p<0.05). Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.
Discussion
Oral carcinogenesis is a multistep process, in which the balance between oncogenes and tumor suppressor genes are altered. Our current data show, for the first time, that DSPP and/MMP20 silencing deregulates major signaling pathways (RAS/RAF, MAPK, NFkB, TGFβ and β Catenin) implicated in OSCC progression. EGFR activates RAS, MAPK, Src, STAT 3/5, PLC, PKC, and PI3 kinase signaling pathways leading to tumor progression [17]. Furthermore, EGFR activates MMPs and STAT3, and facilitates cell invasion by inducing epithelial mesenchymal transition (EMT) [18-20]. Upregulation of EGFR has been reported in several epithelial cancers, including breast, bladder, ovary, renal, colon, and HNSCCs, including OSCCs [21]. Indeed, EGFR overexpression remains an early event in HNSCC, and increased expression has been demonstrated in cultured human HNSCC cells and in corresponding tumor samples [22]. Published reports indicate that more than 90% of patients with HNSCC overexpressing EGFR show accelerated clinical progression, increased resistance to chemotherapy, and an overall low survival rates [23]. Increased EGFR expression also has been suggested as a predictor of neoplastic potential in oral premalignant lesions (OPLs) [24]. Consistent with these reports, the present study showed increased EGFR expression in OSCC cells, (OSC2). Significantly, DSPP/MMP20 silencing in OSC2 cells downregulated EGFR expression, most notably in DSPP-silenced OSC2 cells and less so in MMP20 and combined DSPP-MMP20 -silenced OSC2 cells, suggesting that DSPP silencing alone results in profound downregulation of EGFR in OSC2 cells (Figure 1a).
As indicated above, activation of the EGFR pathway, in turn, leads to activation of three other major pathways: RAF-MEK-ERK, RalGEF-Ral, and PI3K-AKT [25]. KRAS, a member of the RAS family of proteins, is mutated in various cancers, including OSCC, where it is mutated in 40-90% of cases [26, 27]. Mutation of KRAS leads to its amplification, which in turn, leads to increased cell proliferation and other progression signals in HNSCC [26]. By extension, the decreased expressions of KRAS, MEK, MAPK, ERK1, and ERK2 observed in our present study, following DSPP/MMP20 silencing (Figure 1), show that silencing effectively prevents downstream signaling from KRAS. Dysregulation of each of these pathways result in upregulation of associated proteins lead to the promotion of one or more hallmarks of tumorigenesis [28]. For example, upregulation of the MEK pathway lead to tumor progression, whereas upregulation of ERK results in activation of several nuclear and cytoplasmic proteins, including transcription factors, that encode for proteins that promote proliferation, survival, and angiogenesis [29, 30]. Indeed, the activation of the RAS and ERK pathways have been reported to enhance tumor progression and angiogenesis [31]. The MAPK pathway is constitutively activated in several cancers, where it promotes tumor progression and metastasis [32, 33]. Furthermore, the p38/MAPK activation has been associated with EMT and subsequent invasion at primary tumors sites [34].
MEEK proteins (MEEK 1, 2, 3 and 4) are regulators of MAPK kinase kinases (MKKs), and their upregulation results in activation of MAPK pathway [35]. MEEK 1 activates and regulates JNK and ERK1/2, MEEK 2 activates JNK and ERK5, MEEK 3 activates ERK5 and p38, and MEEK4 regulates the activation of JNK and p38 [36]. Like MEK, MEEK1 is upregulated in almost all cancers where it controls cell migration by regulating the formation of focal adhesion kinase [37]. Downregulation or inhibition of MEEK1 has been reported to result in decreased invasion and metastasis and increased apoptosis of cancer cells [37, 38]. Our current findings of the downregulatory effects of DSPP, MMP20, or combined DSPP-MMP20 silencing on MEEK1 (Figure 2a) and JNK (Figure 2b) mirror these previously published results. As shown in (Figure 2), CREB binding protein (CREBP) and its paralog p300 were significantly downregulated following DSPP/MMP20 silencing in OSC2 cells (Figures 2a, 2d). CREBP, an oncogenic transcription factor, regulates a range of biological process from cell growth to differentiation [39]. It is overexpressed in several cancers, including acute lymphoblastic leukemia, osteosarcoma, non small cell lung cancers, cholangiocarcinoma, breast cancer, renal cancer, esophageal cancer, and HNSCC [40-42]. Furthermore, increased expression of CREBBP, EP300, and MMP9 has been reported in OSCC, and upregulation in esophageal squamous cell carcinomas has been associated with lymph node metastasis [43, 44]. CREBP upregulation increased cell migration, invasion, and metastasis via upregulation of MMP2 and MMP9 [45]. With signals originating from pathways such as MAPK and PKA, phosphorylated CREBP binds to histone acetyl transferase, CREB-binding protein (CBP) and p300, to initiate CREB-dependent transcription of genes that aid in cell survival and tumorigenesis [46]. On the other hand, downregulation of CREBP has been reported to trigger apoptosis and cell cycle arrests and decrease metastasis [47].
The nuclear factor-kB (NF-kB) comprises five family members: p65 (RelA), RelB, c-Rel, p50, and p52. They exist as homo and hetero dimers bound to their inhibitory proteins, IκB and IκBα in the cytoplasm [48]. Activation of NF-kB has been reported in melanoma, pancreatic, bladder, breast, and head and neck cancers, including OSCC [49, 50]. Furthermore, reports indicate that upregulation of NF-kB in OSCC increased stemness and was associated with poorly differentiated tumors and worse prognosis [51]. Upregulation of the p50 and p65 subunits in squamous cell carcinoma of the tongue were associated with tumor aggressiveness [52]. NF-kB regulates angiogenesis through the production of VEGF, activates adhesion molecules like ICAM-1, ELAM-1, and VCAM-1, induces cell invasion by the production of MMP’s and inhibits apoptosis [53]. In HNSCC, increased expression of NF-kB induced the expression of MMP 9 and VEGF [50]. While signals that activate NF-kB led to increased levels of p50 and p65 subunits, it also decreased the levels of regulatory (IKKγ) and catalytic (IKKα and IKKβ) subunits of IKK. Inhibiting NF-kB pathway has been reported slow the process of tumorigenesis [54]. Our present data indicates that, while DSPP/MMP20 silencing results in decreased levels of the p50 and p65 subunits, the levels of the catalytic subunit, IKKα, is significantly increased (Figure 3b). It is therefore reasonable to infer that increased levels of IKKα following DSPP/MMP20 silencing in OSC2 cells serves to impede such activities as angiogenesis and other hallmarks of tumorigenesis via downregulation of the p50 and p65 subunits of NF-kB [13]. Furthermore, we speculate that a potential mechanism accounting for increased IKKα levels and decreased p50 and p65 levels following DSPP/MMP20 silencing involves inhibition of NF-kB translocation from cytosol to the nucleus by IKKα, which in turn inhibits the expression of genes involved in proliferation, invasion, and apoptosis.
Dysregulated TGF-β signaling has been reported in many cancers including HNSCC [55]. Mutations in TGFβRII occurs in 21% of OSCC patients, and is associated with increased cell proliferation and decreased apoptosis [56, 57]. The role of TGFβ in tumorigenesis is less straightforward than that of other signaling pathways because of its dual but contradictory roles as a tumor suppressor in the early stages and as an “oncogene” at later stages [58]. Through a series of preceding interactions, TGFβ activates cytosolic Smad proteins (Smad 2 and 3) via phosphorylation of the serine at the C terminal [59]. Phosphorylated Smad 2 and 3 then provide a docking site for Smad 4 [60]. As heteromeric complex, SMADs translocate to nucleus where it binds to SMAD-response element (SREs), to bring about the transcription of genes that facilitate EMT and metastasis [61]. Signaling pathways such as SMAD, PI3K, Rho and MAPK synergize with TGFβ to promote proliferation and invasion of cancer cells [62]. The significantly decreased TGF-β levels, notably with silencing of DSPP or MMP20 singly, compared with combined DSPP-MMP20 silencing, seen in our current data suggests that DSPP/MMP20 interference may abrogate the tumorigenic activities of TGF-β signaling. Complete absence or decreased SMAD4 levels have been reported in various cancers, including HNSCC. Hernandez et al. reported heterozygous loss of SMAD4 in 35% of primary HNSCCs and 41.3% of patient derived xenografts [63].
Loss of SMAD4 in HNSCC patients correlated with pathological stage, regional metastasis, and survival [64]. Significantly, SMAD 4 depletion in HNSCC cell line induced cetuximab resistance through JNK and MAPK activation, and in an orthotopic mouse model resulted in poor survival rate [65]. Given its anti-tumor role, our current data showing that DSPP/MMP20 silencing, individually or in combination, resulted in upregulation of SMAD4 in OSC2 cells, suggests that SMAD4 antitumor effects is enhanced through DSPP/MMP20 silencing. Recent reports show that SMAD7 can mediate TGFβ independent signaling [66]. Increased SMAD7 expression was associated with anchorage-independent cell growth, decreased apoptosis, distant metastasis, and poor survival rates in esophageal squamous cell carcinoma patients [67]. In pancreatic cancer, high expression of SMAD7 induced premalignant ductal lesions, and in gastric cancer patients, increased expression was associated with increased tumor size, depth of invasion, regional lymph node metastasis, and poor prognosis [68]. Increased expression of SMAD7 in HNSCC cell line, correlated with tumor invasion [69]. Thus, we anticipate that decreased levels of SMAD7 seen in our present study, following DSPP/MMP20 silencing, inhibits the various hallmarks of oral carcinogenesis and overall tumor progression.
GSK-3β functions either as a tumor suppressor or promoter, depending on the nature of signal it receives from the mammalian target of rapamycin (mTOR), a protein that plays a crucial role in cell proliferation [70]. GSK-3β is a multifunctional serine/threonine kinase, that regulates several physiological responses. It plays an essential role in WNT signaling pathway by phosphorylating β catenin on key residues [71]. GSK-3β is phosphorylated by PKA, AKT, PKC and MAPK, all of which are activated in OSCC [72]. GSK-3β is overexpressed in various cancers such as colon, liver, ovary, and pancreas. It is also upregulated in OSCCs with its upregulation associated with late stage (Stages III and IV) disease and poor patients survival rates [73]. On the other hand, inhibition of GSK3α/β by SB 216763 in HNSCC cell line, not only led to significant growth inhibition and tumor cell migration, but also reversed changes in EMT [74]. Its downregulation inhibits growth, angiogenesis, and vascular endothelial growth factor expression in pancreatic cancers [75]. β-Catenin upregulation in OSCC has been correlated with increased tumor invasiveness [76].
In patients with oropharyngeal cancers, increased levels of cytoplasmic β-Catenin correlated with poor histologic grade, advanced stage disease, and poor prognosis [77]. Activation of Wnt/β-catenin pathway has been reported to play an important role in maintaining stemness evidenced by increased expression of cancer stem cells (CSC) surface markers, such as LGR5/GPR49, CD44, CD24, and Epcam [78]. Furthermore, it regulates EMT by inducing loss of E-Cadherin, while promoting expression of other EMT genes. It also aids in tumor invasion, by increasing the expression of MMPs [79]. In oral epithelial dysplasia, expression of β-catenin increased with epithelial dysplasia and can be used as a marker when normal oral mucosa turns to oral epithelial dysplasia in oral leukoplakia [80]. Activation of Wnt pathway also leads to the expression of Dickkopf 1 (DKK1), a negative feedback regulator, which in turn suppresses Wnt pathway signaling by interfering with the LRP5/6 co-receptors [81]. HNSCC patients with DKK1 mutations had lower distant metastasis rate and longer disease-free survival rate [81]. Our current data show increased expression of DKK1 following DSPP/MMP20 silencing. Overall, our data showing that DSPP/MMP20 silencing, singly or in combinations, resulted in near complete abrogation of β-catenin suggest that the various tumorigenic activities of β-catenin may be reversed following DSPP/MMP20 silencing.
Our results/data above include instances where either DSPP or MMP20 silencing had more impact on the levels (downregulation versus upregulation) of certain proteins. These data suggest that, although DSPP and MMP20 are cognate partners, they may not necessarily act synergistically in all settings [82]. Indeed, we anticipate that in some settings one may act to compensate for the other, whereas in other settings their actions may be complementary. Furthermore, our results indicating a greater singular effect of DSPP silencing compared with MMP20 and combined MMP20-DSPP silencing suggests that MMP20 silencing may obstruct the effect of DSPP single silencing. These possibilities are design subjects of our next study beyond the scope of our present study. Although the use of a single cell line and the data, based on western blot analyses alone, presents inherent limitations, the overall conclusions are significant and provide solid and logical basis for further studies as indicated above.
Conclusion
While our published reports show that DSPP and its cognate MMP20 are upregulated in OSCCs, our current report shows that DSPP/MMP20 silencing affects key signaling pathways widely implicated in notable hallmarks of oral malignancy: cell growth, increased invasiveness and migration, and metastasis. Based on our current data, we propose a network and action points on the various pathways affected by the abrogation of DSPP and MMP20 that may account for the results in this study. This is summarized in (Figure 6) schematic illustration. Ongoing studies beyond the scope of the present report will aim to verify that DSPP/MMP20 silencing results in decreased cell growth and proliferation, decreased invasion and migration in OSCC cells, and other clinical indicia of oral malignancy. Significantly, our findings that DSPP/MMP20 silencing in OSC2 cells resulted in the downregulation of genes known to promote oral tumorigenesis and the upregulation of genes known to suppress oral tumorigenesis via a “global” effect on multiple signaling pathways provides promise for potential therapeutic interventions. Such intervention will target DSPP/MMP20 downregulation to abrogate multiple tumorigenic pathways rather than a single or few of the pathways.
Figure 6: Schematic summary of proposed network and action points (red ovals) of the effects of DSPP, MMP20 Silencing on RTKs, TLR, TGF Beta, and Wnt pathways.
Acknowledgements
None.
Funding
University of Texas Health Science Center at Houston Startup research funding for KUO.
Availability of Data and Materials
None. All data are part the current submission.
Author Contributions
J.A.: concept: designed, performed experiments, generated draft manuscript; reviewed final draft manuscript. K.U.E.O.: concept: designed; edited draft manuscripts; reviewed final draft manuscript. All authors have read and agreed to the published version of the manuscript.
Ethics Approval and Consent to Participate
None.
Patient Consent for Publication
None.
Competing Interests
None.
Authors' Information
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 13, Jun 2023Accepted: Wed 28, Jun 2023
Published: Tue 12, Sep 2023
Copyright
© 2023 Kalu U.E. Ogbureke. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2023.02.02
Author Info
Jaya Aseervatham Kalu U.E. Ogbureke
Corresponding Author
Kalu U.E. OgburekeDepartment of Diagnostic and Biomedical Sciences, School of Dentistry, University of Texas Health Science Center at Houston, Houston, Texas, USA
Figures & Tables

A) The protien expression of EGFR decreased by 96% in shDSPP cells, 46% in shMMP20 cells (p<0.05), and 67% in shDM cells (p< 0.01), compared with control. B) KRAS decreased significantly by 52%, 59 and 49% in shDSPP, shMMP20 and shDM cells, respectively (p<0.001), compared with shC. C) The expression of MEK1/MEK2 decreased by 33%, 34% and 30% in shDSPP, shMMP20 and shDM cells, respectively (p<0.05 and p<0.01), compared with shC. D) MAPK levels decreased by 37% in shDSPP (p<0.05), 48% in shMMP20 (p<0.01) and 62% in shDM cells (p<0.01) compared with shC. E) ERK1/ERK2 levels decreased by 76% in shDSPP, 73% in shMMP20, and by 64% in shDM (p< 0.001) compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.

a) MEEK1 protein expression decreaed by 72% in shDSPP cells (p<0.001), 50% in shMMP20 cells (p<0.01), and 48% in shDM cells (p< 0.01), compared with ShC. b) JNK decreased significantly by 47% (p<0.01), 33%, (p<0.05), and 42% (p<0.05) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. c) The expression of CREBP decreased by 72% (p<0.01), 76% (p<0.01), and 48% (p<0.05) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. d) p300 levels decreased by 58% in shDSPP (p<0.01), 45% in shMMP20 (p<0.05) and 62% in shDM cells (p<0.01) compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.

A) Protein expression of NFkB p50 subunit decreased by 46% in shDSPP cells (p<0.05), 39% in shMMP20 cells (p<0.01), and 41% in shDM cells (p< 0.05), compared to control. B) p65 subunit of NFkB decreased significantly by 74% (p<0.05), 56%, (p<0.05), and 55% (p<0.01) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. C) The expression of IKKα increased by 2-fold (p<0.01), 1.7-fold (p<0.01), and 2.1-fold (p<0.01) in shDSPP, shMMP20 and shDM cells respectively, compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.

A) Protein expression of TGFβ decreased by 55% in shDSPP cells (p<0.01), 30% in shMMP20 cells (p<0.05), and 53% in shDM cells (p< 0.01), compared with ShC. B) SMAD7 levels decreased significantly by 47% (p<0.01), 74%, (p<0.05), and 63% (p<0.05) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. C) The levels of SMAD4 increased by 1.5-fold (p<0.01), 1.7-fold (p<0.01), and 2.0-fold (p<0.001) in shDSPP, shMMP20 and shDM cells, respectively, compared with shC. Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 Silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.
A) Protein expression of GSK3β decreased by 65% and 64% and 41% respectively in the DSPP, MMP20 and DM group (p<0.01) (p<0.05). B) The expression of β catenin was almost nil in all the groups under investigation (97%, 99% and 92% in the DSPP, MMP20 and DM group (p<0.001). C) The expression of DKK1 increased by 1.7-fold in the DSPP silenced group and 1.6- fold in the MMP20 and DM group (p<0.05). Beta actin was used as the internal control. Values are given as mean ± SE for 3 independent experiments.
shC: Scrambled Control; shDSPP: DSPP Silenced OSC2 cells; shMMP20: MMP20 silenced OSC2 cells; DM: Combined DSPP-MMP20 Silenced OSC2 cells.
*p < 0.05; ** p < 0.01; *** p <0.001.

References
1.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE et al. (2020) Head
and neck squamous cell carcinoma. Nat Rev Dis Primers 6: 92. [ Crossref]
2.
Fejza A, Camicia L, Poletto E, Carobolante G, Mongiat M et al. (2021) ECM
Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for
Cancer Dissemination and Emerging Biomarkers. Cancers (Basel) 13: 2759.
[ Crossref]
3.
Kariche N, Hortal MT, Benyahia S, Alemany L, Moulai N et al. (2018)
Comparative assessment of HPV, alcohol and tobacco etiological fractions in
Algerian patients with laryngeal squamous cell carcinoma. Infect Agent
Cancer 13: 8. [ Crossref]
4.
Abati S, Bramati C, Bondi S, Lissoni A, Trimarchi M (2020) Oral Cancer and
Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int J
Environ Res Public Health 17: 9160. [ Crossref]
5.
Aittiwarapoj A, Juengsomjit R, Kitkumthorn N, Lapthanasupkul P (2019) Oral
Potentially Malignant Disorders and Squamous Cell Carcinoma at the Tongue:
Clinicopathological Analysis in a Thai Population. Eur J Dent 13:
376-382. [ Crossref]
6.
Wang J, Cui R, Clement CG, Nawgiri R, Powell DW et al. (2020) Activation
PDGFR-α/AKT Mediated Signaling Pathways in Oral Squamous Cell Carcinoma by
Mesenchymal Stem/Stromal Cells Promotes Anti-apoptosis and Decreased
Sensitivity to Cisplatin. Front Oncol 10: 552. [ Crossref]
7.
Aseervatham J, Geetu S, Anunobi CC, Koli K, Ogbureke KUE (2019) Survey of
dentin sialophosphoprotein and its cognate matrix metalloproteinase-20 in human
cancers. Cancer Med 8: 2167-2178. [ Crossref]
8.
Bao Q, Zhang J, Wang XX (2018) Effects of Btbd7 knockdown on the
proliferation of human dental pulp cells and expression of Dspp. Int J Clin
Exp Pathol 11: 1460-1465. [ Crossref]
9.
Fan S, Gao H, Sun L, Zhu F, Zhou R et al.: Knockdown of DSPP inhibits the
migration and invasion of glioma cells. Pathol Res Pract 214: 2025-2030.
[ Crossref]
10.
Hamilton SL, Ferando B, Eapen AS, Yu JC, Joy AR (2017) Cancer Secretome May
Influence BSP and DSP Expression in Human Salivary Gland Cells. J Histochem
Cytochem 65: 139-151. [ Crossref]
11.
Joshi R, Tawfik A, Edeh N, McCloud V, Looney S et al. (2010) Dentin
sialophosphoprotein (DSPP) gene-silencing inhibits key tumorigenic activities
in human oral cancer cell line, OSC2. PLoS One 5: e13974. [ Crossref]
12.
Ogbureke KU, Fisher LW (2007) SIBLING expression patterns in duct epithelia
reflect the degree of metabolic activity. J Histochem Cytochem 55:
403-409, 2007. [ Crossref]
13.
Aseervatham J, Ogbureke KUE (2020) Effects of DSPP and MMP20 Silencing on Adhesion,
Metastasis, Angiogenesis, and Epithelial-Mesenchymal Transition Proteins in
Oral Squamous Cell Carcinoma Cells. Int J Mol Sci 21: 4734. [ Crossref]
14.
Nikitakis NG, Gkouveris I, Aseervatham J, Barahona K, Ogbureke KUE (2018)
DSPP-MMP20 gene silencing downregulates cancer stem cell markers in human oral
cancer cells. Cell Mol Biol Lett 23: 30. [ Crossref]
15.
Hsu JL, Hung MC (2016) The role of HER2, EGFR, and other receptor tyrosine
kinases in breast cancer. Cancer Metastasis Rev, 35: 575-588. [ Crossref]
16.
Zolghadr F, Tse N, Loka D, Joun G, Meppat S et al. (2021) A Wnt-mediated
phenotype switch along the epithelial-mesenchymal axis defines resistance and
invasion downstream of ionising radiation in oral squamous cell carcinoma. Br
J Cancer 124: 1921-1933. [ Crossref]
17.
Sigismund S, Avanzato D, Lanzetti L (2018) Emerging functions of the EGFR
in cancer. Mol Oncol 12: 3-20. [ Crossref]
18.
Majumder A, Ray S, Banerji A (2019) Epidermal growth factor
receptor-mediated regulation of matrix metalloproteinase-2 and matrix
metalloproteinase-9 in MCF-7 breast cancer cells. Mol Cell Biochem 452:
111-121. [ Crossref]
19.
Jackson NM, Ceresa BP (2017) EGFR-mediated apoptosis via STAT3. Exp Cell
Res 356: 93-103. [ Crossref]
20.
Wendt MK, Balanis N, Carlin CR, Schiemann WP (2014) STAT3 and epithelial-mesenchymal
transitions in carcinomas. Jakstat 3: e28975. [ Crossref]
21.
Han W, Lo HW (2012) Landscape of EGFR signaling network in human cancers:
biology and therapeutic response in relation to receptor subcellular locations.
Cancer Lett 318: 124-134. [ Crossref]
22.
Byeon HK, Ku M, Yang J (2019) Beyond EGFR inhibition: multilateral combat
strategies to stop the progression of head and neck cancer. Exp Mol Med
51: 1-14. [ Crossref]
23.
Cassell A, Grandis JR (2010) Investigational EGFR-targeted therapy in head
and neck squamous cell carcinoma. Expert Opin Investig Drugs 19:
709-722. [ Crossref]
24.
Meka NJ, Ugrappa S, Velpula N, Kumar S, Malth KN et al. (2015) Quantitative
Immunoexpression of EGFR in Oral Potentially Malignant Disorders: Oral
Leukoplakia and Oral Submucous Fibrosis. J Dent Res Dent Clin Dent Prospects
9: 166-174. [ Crossref]
25.
Eser S, Schnieke A, Schneider G, Saur D (2014) Oncogenic KRAS signalling in
pancreatic cancer. Br J Cancer 111: 817-822. [ Crossref]
26.
Sasaki E, Masago K, Fujita S, Hanai N, Yatabe Y (2020) Frequent KRAS and
HRAS mutations in squamous cell papillomas of the head and neck. J Pathol
Clin Res 6: 154-159. [ Crossref]
27.
Weidhaas JB, Harris J, Schaue D, Chen AM, Chin R et al. (2017) The
KRAS-Variant and Cetuximab Response in Head and Neck Squamous Cell Cancer: A
Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 3:
483-491. [ Crossref]
28.
Usman S, Jamal A, Teh MT, Waseem A (2021) Major Molecular Signaling
Pathways in Oral Cancer Associated With Therapeutic Resistance. Front Oral
Health 1: 603160. [ Crossref]
29.
Neuzillet C, Tijeras Raballand A, de Mestier L, Cros J, Faivre S et al.
(2014) MEK in cancer and cancer therapy. Pharmacol Ther 141: 160-171. [ Crossref]
30.
Maik Rachline G, Hacohen Lev Ran A, Seger R (2019) Nuclear ERK: Mechanism
of Translocation, Substrates, and Role in Cancer. Int J Mol Sci 20:
1194. [ Crossref]
31.
Peng Q, Deng Z, Pan H, Gu L, Liu O et al. (2018) Mitogen-activated protein
kinase signaling pathway in oral cancer. Oncol Lett 15: 1379-1388. [ Crossref]
32.
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y et al. (2020) ERK/MAPK signalling
pathway and tumorigenesis. Exp Ther Med 19: 1997-2007. [ Crossref]
33.
Burotto M, Chiou VL, Lee JM, Kohn EC (2014) The MAPK pathway across
different malignancies: a new perspective. Cancer 120: 3446-3456. [ Crossref]
34.
Huang Q, Lan F, Wang X, Yu Y, Ouyang X et al. (2014) IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer 13: 18. [ Crossref]
35.
Yuan J, Dong X, Yap J, Hu J (2020) The MAPK and AMPK signalings: interplay
and implication in targeted cancer therapy. J Hematol Oncol 13: 113. [ Crossref]
36.
Su F, Li H, Yan C, Jia B, Zhang Y et al. (2009) Depleting MEKK1 expression
inhibits the ability of invasion and migration of human pancreatic cancer
cells. J Cancer Res Clin Oncol 135: 1655-1663. [ Crossref]
37.
Cuevas BD, Winter Vann AM, Johnson NL, Johnson GL (2006) MEKK1 controls
matrix degradation and tumor cell dissemination during metastasis of polyoma
middle-T driven mammary cancer. Oncogene 25: 4998-5010. [ Crossref]
38.
Lu H, Ning X, Tao X, Ren J, Song X et al. (2016) MEKK1 Associated with
Neuronal Apoptosis Following Intracerebral Hemorrhage. Neurochem Res 41:
3308-3321. [ Crossref]
39.
Friedrich M, Heimer N, Stoehr C, Steven A, Wach S et al. (2020) CREB1 is
affected by the microRNAs miR-22-3p, miR-26a-5p, miR-27a-3p, and miR-221-3p and
correlates with adverse clinicopathological features in renal cell carcinoma. Sci
Rep 10: 6499. [ Crossref]
40.
Martin D, Abba MC, Molinolo AA, Vitale Cross L, Wang Z et al. (2014) The
head and neck cancer cell oncogenome: a platform for the development of
precision molecular therapies. Oncotarget 5: 8906-8923. [ Crossref]
41.
van der Sligte NE, Kampen KR, ter Elst A, Scherpen FJG, Meeuwsen de Boer
TGJ et al. (2015) Essential role for cyclic-AMP responsive element binding
protein 1 (CREB) in the survival of acute lymphoblastic leukemia. Oncotarget
6: 14970-14981. [ Crossref]
42.
Wang YW, Chen X, Gao JW, Zhang H, Ma RR et al. (2015) High expression of
cAMP-responsive element-binding protein 1 (CREB1) is associated with
metastasis, tumor stage and poor outcome in gastric cancer. Oncotarget
6: 10646-10657. [ Crossref]
43.
Yeh CM, Lin CW, Yang JS, Yang WE, Su SC et al. (2016) Melatonin inhibits
TPA-induced oral cancer cell migration by suppressing matrix
metalloproteinase-9 activation through the histone acetylation. Oncotarget
7: 21952-21967. [ Crossref]
44.
Sawada G, Niida A, Uchi R, Hirata H, Shimamura T et al. (2016) Genomic
Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology
150: 1171-1182. [ Crossref]
45.
Wang X, Cui H, Lou Z, Huang S, Ren Y et al. (2017) Cyclic AMP responsive
element-binding protein induces metastatic renal cell carcinoma by mediating
the expression of matrix metallopeptidase-2/9 and proteins associated with
epithelial-mesenchymal transition. Mol Med Rep 15: 4191-4198. [ Crossref]
46.
Dancy BM, Cole PA (2015) Protein lysine acetylation by p300/CBP. Chem
Rev 115: 2419-2452. [ Crossref]
47.
Chen P, Li M, Hao Q, Zhao X, Hu T (2018) Targeting the overexpressed CREB
inhibits esophageal squamous cell carcinoma cell growth. Oncol Rep 39:
1369-1377. [ Crossref]
48.
Giuliani C, Bucci I, Napolitano G (2018) The Role of the Transcription
Factor Nuclear Factor-kappa B in Thyroid Autoimmunity and Cancer. Front
Endocrinol (Lausanne) 9: 471. [ Crossref]
49.
Xia Y, Shen S, Verma IM (2014) NF-κB, an active player in human cancers. Cancer
Immunol Res 2: 823-830. [ Crossref]
50.
Yan M, Xu Q, Zhang P, Zhou XJ, Zhang ZY et al. (2010) Correlation of
NF-kappaB signal pathway with tumor metastasis of human head and neck squamous
cell carcinoma. BMC Cancer 10: 437. [ Crossref]
51.
Lakshminarayana S, Augustine D, Rao RS, Patil S, Awan KH et al. (2018)
Molecular pathways of oral cancer that predict prognosis and survival: A
systematic review. J Carcinog 17: 7. [ Crossref]
52.
Gupta S, Kumar P, Kaur H, Sharma N, Gupta S et al. (2018) Constitutive
activation and overexpression of NF-κB/c-Rel in conjunction with p50 contribute
to aggressive tongue tumorigenesis. Oncotarget 9: 33011-33029. [ Crossref]
53.
Concetti J, Wilson CL (2018) NFKB1 and Cancer: Friend or Foe? Cells
7: 133. [ Crossref]
54.
Yu H, Lin L, Zhang Z, Zhang H, Hu H (2020) Targeting NF-κB pathway for the
therapy of diseases: mechanism and clinical study. Signal Transduct Target
Ther 5: 209. [ Crossref]
55.
Malkoski SP, Wang XJ (2012) Two sides of the story? Smad4 loss in
pancreatic cancer versus head-and-neck cancer. FEBS Lett 586: 1984-1992.
[ Crossref]
56.
Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K et al. (2011)
Exome sequencing of head and neck squamous cell carcinoma reveals inactivating
mutations in NOTCH1. Science 333: 1154-1157. [ Crossref]
57.
Wu F, Weigel KJ, Zhou H, Wang XJ (2018) Paradoxical roles of TGF-β
signaling in suppressing and promoting squamous cell carcinoma. Acta Biochim
Biophys Sin (Shanghai) 50: 98-105. [ Crossref]
58.
Seoane J, Gomis RR (2017) TGF-β Family Signaling in Tumor Suppression and
Cancer Progression. Cold Spring Harb Perspect Biol 9: a022277. [ Crossref]
59.
Aragón E, Wang Q, Zou Y, Morgani SM, Ruiz L et al. (2019) Structural basis
for distinct roles of SMAD2 and SMAD3 in FOXH1 pioneer-directed TGF-β
signaling. Genes Dev 33: 1506-1524. [ Crossref]
60.
Liu L, Liu X, Ren X, Tian Y, Chen Z et al. (2016) Smad2 and Smad3 have
differential sensitivity in relaying TGFβ signaling and inversely regulate
early lineage specification. Sci Rep 6: 21602. [ Crossref]
61.
Zhao M, Mishra L, Deng CX (2018) The role of TGF-β/SMAD4 signaling in
cancer. Int J Biol Sci 14: 111-123. [ Crossref]
62.
Luo K (2017) Signaling Cross Talk between TGF-β/Smad and Other Signaling
Pathways. Cold Spring Harb Perspect Biol 9: a022137. [ Crossref]
63.
Hernandez AL, Wang Y, Somerset HL, Keysar SB, Aisner DL et al. (2019)
Inter- and intra-tumor heterogeneity of SMAD4 loss in head and neck squamous
cell carcinomas. Mol Carcinog 58: 666-673. [ Crossref]
64.
Lin LH, Chang KW, Cheng HW, Liu CJ (2019) SMAD4 Somatic Mutations in Head
and Neck Carcinoma Are Associated With Tumor Progression. Front Oncol 9:
1379. [ Crossref]
65.
Ozawa H, Ranaweera RS, Izumchenko E, Makarev E, Zhavoronkov A et al. (2017)
SMAD4 Loss Is Associated with Cetuximab Resistance and Induction of MAPK/JNK
Activation in Head and Neck Cancer Cells. Clin Cancer Res 23: 5162-5175.
[ Crossref]
66.
Miyazawa K, Miyazono K (2017) Regulation of TGF-β Family Signaling by
Inhibitory Smads. Cold Spring Harb Perspect Biol 9: a022095. [ Crossref]
67.
Stolfi C, Marafini I, De Simone V, Pallone F, Monteleone G (2013) The dual
role of Smad7 in the control of cancer growth and metastasis. Int J Mol Sci
14: 23774-23790. [ Crossref]
68.
Luo L, Li N, Lv N, Huang D (2014) SMAD7: a timer of tumor progression
targeting TGF-β signaling. Tumour Biol 35: 8379-8385. [ Crossref]
69.
Luo J, Bian L, Blevins MA, Wang D, Liang C et al. (2019) Smad7 Promotes
Healing of Radiotherapy-Induced Oral Mucositis without Compromising Oral Cancer
Therapy in a Xenograft Mouse Model. Clin Cancer Res 25: 808-818. [ Crossref]
70.
Duda P, Akula SM, Abrams SL, Steelman LS, Martelli AM et al. (2020)
Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells
9: 1110. [ Crossref]
71.
McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Abrams SL et al. (2014)
Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and
leukemogenesis: opportunities for therapeutic intervention. Leukemia 28:
15-33. [ Crossref]
72.
Medina M, Wandosell F (2011) Deconstructing GSK-3: The Fine Regulation of
Its Activity. Int J Alzheimers Dis 2011: 479249. [ Crossref]
73.
Mishra R, Nagini S, Rana A (2015) Expression and inactivation of glycogen
synthase kinase 3 alpha/ beta and their association with the expression of cyclin
D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer 14:
20. [ Crossref]
74.
Schulz L, Pries R, Lanka AS, Drenckhan M, Rades D et al. (2018) Inhibition
of GSK3α/β impairs the progression of HNSCC. Oncotarget 9: 27630-27644.
[ Crossref]
75.
He R, Du S, Lei T, Xie X, Wang Y (2020) Glycogen synthase kinase 3β in
tumorigenesis and oncotherapy (Review). Oncol Rep 44: 2373-2385. [ Crossref]
76.
González-Moles MA, Ruiz-Ávila I, Gil-Montoya JA, Plaza-Campillo J, Scully C
(2014) β-catenin in oral cancer: an update on current knowledge. Oral Oncol
50: 818-824. [ Crossref]
77.
Hu Z, Müller S, Qian G, Xu J, Kim S et al. (2015) Human papillomavirus 16
oncoprotein regulates the translocation of β-catenin via the activation of
epidermal growth factor receptor. Cancer 121: 214-225. [ Crossref]
78.
Mohammed MK, Shao C, Wang J, Wei Q, Wang X et al. (2016) Wnt/β-catenin
signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis
and cancer chemoresistance. Genes Dis 3: 11-40. [ Crossref]
79.
Mylavarapu S, Kumar H, Kumari S, Sravanthi LS, Jain M et al. (2019)
Activation of Epithelial-Mesenchymal Transition and Altered β-Catenin Signaling
in a Novel Indian Colorectal Carcinoma Cell Line. Front Oncol 9: 54. [ Crossref]
80.
Kaur J, Sawhney M, DattaGupta S, Shukla NK, Srivastava A et al. (2013)
Clinical significance of altered expression of β-catenin and E-cadherin in oral
dysplasia and cancer: potential link with ALCAM expression. PLoS One 8:
e67361. [ Crossref]
81. Katase N, Gunduz M, Beder LB, Gunduz E, Ali MAS et al. (2010) Frequent allelic loss of Dkk-1 locus (10q11.2) is related with low distant metastasis and better prognosis in head and neck squamous cell carcinomas. Cancer Invest 28: 103-110. [ Crossref]
82. Saxena G, Koli K, de la Garza J, Ogbureke KU (2015) Matrix metalloproteinase 20-dentin sialophosphoprotein interaction in oral cancer. J Dent Res 94: 584-593. [ Crossref]
