Journals
Effect of reactive oxygen species on the ligand-independent activation of EGFR in tongue squamous cell carcinoma
A B S T R A C T
Objective: To explore the expression of 8-hydroxy-2′-deoxyguanosine (8-OHdG), p-Src, and phosphor-epidermal growth factor receptor (p-EGFR) and the effect of reactive oxygen species on ligand-independent activation of EGFR in tongue squamous cell carcinoma (TSCC) tissues.
Methods: The expressions of 8-OHdG, EGFR, and p-EGFR in carcinoma tissues and carcinoma-adjacent tissues of 40 cases of TSCC were determined immunohistochemically. The correlations between 8-OHdG expression and p-Src and p-EGFR expressions were analyzed.
Results: 8-OHdG, p-Src, and p-EGFR were highly expressed in TSCC tissues, with positivity rates of 82.5%, 70%, and 55%, respectively, whereas they were lowly expressed in carcinoma-adjacent tissues, with positivity rates of 17.5%, 12.5%, and 7.5%, respectively. The expressions of 8-OHdG, p-Src, and p-EGFR were significantly higher in carcinoma tissue than in carcinoma-adjacent tissue (P < 0.05), and statistically significant correlations were observed between 8-OHdG expression and p-Src and p-EGFR expressions (P < 0.05).
Conclusion: This study preliminarily determined the expression of the reactive oxygen species-Src-EGFR signaling pathway in TSCC, providing clinical evidence for the use of cetuximab in combination with other chemotherapy drugs in the treatment of oral cancer.
Keywords
ROS p-Src, p-EGFR, TSCC, Cetuximab
I N T R O D U C T I O N
Oral cancer, a malignant tumor with a high incidence, is the sixth-most common cancer worldwide. Among oral cancers, tongue squamous cell carcinoma (TSCC) is the most frequent type of oral squamous cell carcinoma (OSCC) [1]. TSCC has a poor prognosis and high mortality rates because of its high rates of proliferation and metastasis [2]. TSCC usually leads to dysfunctions in speech, mastication, and deglutition. Although recent developments have improved the therapeutic management of TSCC, its recurrence and mortality remain a crucial impediment to treatment [3]. Therefore, understanding molecular pathways and identifying molecular biomarkers of TSCC progression may facilitate early detection and provide novel therapeutic strategies for TSCC.
The epidermal growth factor receptor (EGFR) is a membrane surface receptor with tyrosine kinase activity. EGFR is recognized by its specific ligands to activate the intracellular tyrosine kinase domain following the induction of the expression of related genes in the nucleus. Accumulating evidence has shown that EGFR is often overexpressed in gastrointestinal and abdominal cancer, lung cancer, melanomas, glioblastomas, and OSCC [4]. Epidermal growth factor (EGF), transforming growth factor-alpha (TGF-alpha), and other specific ligands can bind to EGFR on the cell surface to induce receptor dimerization, tyrosine kinase activation, cross-phosphorylation (p-EGFR), and further stimulate downstream signaling proteins to initiate several signal transduction cascades. Signal transduction mediated by EGFR tyrosine kinase is closely associated with tumor proliferation, angiogenesis, tumor invasion and metastasis, and inhibition of cell apoptosis [5].
EGFR has been investigated as a target for the treatment of uncontrolled tumor growth. Preclinical studies suggest that the monoclonal antibody cetuximab can competitively prevent specific ligands from binding to EGFR and inhibit its activity; thus, cetuximab has shown treatment efficacy in advanced head and neck cancer [6]. Unfortunately, only 10-20% of cancer patients are responsive to and clinically benefit from anti-EGFR monoclonal antibody due to intrinsic as well as acquired resistance [7]. In addition, apart from ligand-dependent EGFR tyrosine activation, ligand-independent EGFR tyrosine activation has also been reported; i.e., reactive oxidative species (ROS) [8, 9]. Research has identified a novel mechanism in which angiotensin II causes chronic transactivation of EGFR through ROS activation of Src, which then phosphorylates EGFR at tyrosine 845, leading to sustained EGFR-ERK signaling and phenotypic changes in renal proximal tubule epithelial cells [10]. Oxidative DNA damage plays crucial roles in the pathogenesis of numerous diseases, including cancer. 8-hydroxy-2′-deoxyguanosine (8-OHdG) is the most representative product of oxidative modifications of DNA and can be used as a biomarker for oral cancer screening [11].
Therefore, the current study used the immunohistochemistry (IHC) SP method to detect the expressions of 8-OHdG, p-Src, and p-EGFR in TSCC and its adjacent tissues; analyzed their relationships and correlations; and preliminarily explored the mechanism of ROS-mediated activation of an EGFR signaling pathway to provide information for further studies on the effectiveness of targeted drug treatments in oral cancer.
Materials and Methods
Patients: This retrospective study of 80 specimens included paired TSCC and carcinoma-adjacent tissues obtained from 40 patients of the stomatological hospital affiliated with Guangxi Medical University in 2010. None of the patients received chemotherapy, radiation therapy, or any previous tumor treatment before undergoing operation in the present study. All 40 patients were treated with extended resection and neck dissection. The primary tumors were resected with a 2.0-cm surgical margin. The patient samples were allocated to the experimental group containing 40 TSCC tissue samples or the control group comprising 40 paired carcinoma-adjacent tissue samples. All 80 specimens were paraffin embedded and assessed by two senior pathology doctors in a double-blind manner.
IHC assay: The antibodies used in the present study included mouse anti-human monoclonal 8-OHdG antibody (Santa Cruz, USA) and p-Src and p-EGFR (Tyr845) rabbit antibodies (BIOSS Biotechnology CO., Ltd. Beijing, China) used in a commercial IHC SP kit (Zhongshan Golden Bridge Biotechnology Co., Ltd. Beijing, China). All antibodies used in the present study were suitable for the detection of proteins in humans. Specimens obtained from patients were paraffin embedded; pathological sectioning was done with 4-μm-thick slices that were deparaffinized and rehydrated. Afterward, the antigens were retrieved by heating the specimens in a microwave. The tissue slides were incubated at 4°C overnight with diluted primary antibodies against 8-OHdG (1:200), p-Src (1:100), and p-EGFR (1:100). The secondary antibody diaminobenzidine, a chromogenic reagent, was then added. Finally, the pathological sections were counterstained with hematoxylin, dehydrated with alcohol, vitrified with dimethylbenzene, and mounted with neutral gum. Negative controls were obtained by using PBS instead of the primary antibody.
Evaluation of the 8-OHdG, p-Src, and p-EGFR results: The sections were assessed in a double-blind manner and examined using the image analyzer of a light microscope at a high magnification. Five fields of each specimen were observed randomly. 8-OHdG, p-Src, and p-EGFR expressions were evaluated based on the percentage of positively stained tumor cells and staining intensity as described previously, with minor modifications as follows: no staining was assigned a score of 0, weak staining, 1; intermediate staining, 2; and strong staining, 3 [12]. If 0% of the cells was stained, the score was 0; 1, < 10 was 1, 11-25% was 2; 26-50% was 3, and 51-100% was 4. According to the product of the two variables, the samples were categorized as follows: negative (-), 0-3; mildly positive (+), 4-6; moderately positive (++), 7-9; and strongly positive (+++), [10-12].
Statistical analysis: The data were processed using IBM SPSS Statistics for Windows, version 19.0. The expressions of 8-OHdG, p-Src, and p-EGFR in TSCC and paired carcinoma-adjacent tissues were compared by Chi-square tests. The correlation analysis was performed using Spearman rank correlation tests. The results were considered statistically significant for P values of < 0.05.
Results
8-OHdG, p-Src, and p-EGFR expressions in TSCC. In TSCC, positive 8-OHdG expression was mainly observed in the nucleus of tumor cells and infiltrating granulocytes, while positive p-Src and p-EGFR expressions were mainly detected in the cytoplasm and cytomembrane of tumor cells (Fig. 1). Among the 40 TSCC tissue samples, 82.5%(33/40) were positive for 8-OHdG expression, 70% (28/40) were positive for p-Src expression, and 55% (22/40) were positive for p-EGFR expression. In comparison, in the carcinoma-adjacent tissues, positive expressions of 8-OHdG, p-Src, and p-EGFR were observed in 17.5% (7/40), 12.5% (5/40), and 7.5% (3/40) of samples, respectively. The expressions of 8-OHdG, p-Src, and p-EGFR were significantly higher in carcinoma tissues than those in carcinoma-adjacent tissues (P < 0.05) (Table 1).
Figure 1: Immunohistochemical staining of 8-OHdG, p-Src, and p-EGFR in TSCC tissues. 8-OHdG expression in carcinoma-adjacent tissue (A and B) and carcinoma tissues (C and D; the black and red arrows in D indicate tumor cells and infiltrating granulocytes, respectively). p-Src expression in carcinoma-adjacent (E and F) and carcinoma (G and H) tissues. p-EGFR expression in carcinoma-adjacent (I and J) and carcinoma (K and L) tissues. SP ×100 (A, C, E, G, I, and K), SP ×400 (B, D, F, H, J, and L).
Tables
Table 1: 8-OHdG, p-Src, and p-EGFR expression in 40 patients with TSCC
|
|
|
|
8-OHdG |
|
|
p-Src |
|
|
p-EGFR |
|
|||
|
Group |
N |
Negative |
Positive |
P |
Negative |
Positive |
P |
Negative |
Positive |
P |
|||
|
TSCC |
40 |
7 |
33 |
<0.05 |
12 |
28 |
<0.05 |
18 |
22 |
<0.05 |
|||
|
Carcinoma-adjacent tissue |
40 |
33 |
7 |
|
35 |
5 |
|
37 |
3 |
|
|||
Correlations between 8-OHdG expression and p-Src and p-EGFR expression. The expression of 8-OHdG was positively correlated with that of p-Src in both samples (r = 0.387, P < 0.05) and was also positively correlated with p-EGFR expression (r = 0.329, P < 0.05) (Spearman rank correlation test, Table 2).
Table 2: Relationship between 8-OHdG expression and p-Src and p-EGFR expressions
|
|
p-Src |
p-EGFR |
||||||||||
|
8-OHdG |
– |
+ |
++ |
+++ |
R |
P |
– |
+ |
++ |
+++ |
R |
P |
|
— |
2 |
2 |
0 |
0 |
0.387 |
0.011 |
4 |
0 |
0 |
0 |
0.329 |
0.038 |
|
+ |
2 |
0 |
1 |
0 |
|
|
2 |
0 |
1 |
0 |
|
|
|
++ |
1 |
1 |
7 |
5 |
|
|
3 |
2 |
6 |
3 |
|
|
|
+++ |
0 |
4 |
8 |
7 |
|
|
2 |
5 |
11 |
1 |
|
|
Discussion
ROS are involved in not only most disease development but also tumor processes. Various experimental and clinical data suggest that ROS are closely related to the biological characteristics of tumors. Sublethal ROS levels are tumorigenic by virtue of their ability to contribute to metastasis, migration, invasion, carcinogenesis, and angiogenesis via lipid peroxidation, DNA damage, and protein injury. However, high ROS levels, which may result from anti-cancer treatment, induces cellular senescence and cell death because of the oxidative nature of the molecules [13]. Saliva and bacteria in the oral cavity increases its susceptibility to infection. Poor oral hygiene, dental prostheses, increased dental calculus, and other chronic inflammation stimulation can stimulate inflammatory cells to produce ROS [14, 15]. Moreover, clinical research has shown that the increased number of neutrophils and macrophages in head and neck squamous cell carcinoma tissues is associated with disease progression and poor prognosis of cancer patients. These two types of cells not only produce a variety of cytokines but also can be activated after contact with tumor cells to release ROS [13, 16, 17]. ROS are powerful oxidizing agents capable of damaging DNA and other biomolecules. Oxidative DNA damage cause increased levels of 8-OHdG and other mutagenic bases in cellular DNA and are associated with increased cancer incidence in humans. 8-OHdG, the major product of oxidative DNA damage, is stable in vivo and can be used as a biomarker for the screening of oral cancer [18]. The present study showed that 8-OHdG was highly expressed in TSCC cells and infiltrating granulocytes and higher than the expression level in paired carcinoma-adjacent tissue, suggesting the important role of macrophages and neutrophil-released ROS in the occurrence and development of TSCC in oral cavity environment and tumor tissues.
Grandis and Tweardy reported upregulation of EGFR mRNA in about 90% of tumors and histologically normal mucosa samples from squamous cell carcinoma of the head and neck patients compared to expression levels in controls [19]. Accumulating evidence has shown that EGFR and its specific ligands (EGF and TGF-alpha) are often overexpressed in oral squamous cell carcinoma, which is closely associated with tumor proliferation, angiogenesis, tumor invasion and metastasis, and inhibition of cell apoptosis [5]. In addition, recent quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analyses demonstrated that PEG-catalase markedly reduced the increased expression of EGFR in the lungs of mice and effectively prevented the growth of metastatic melanomas by detoxifying the ROS [20]. ROS obviously participates in EGFR tyrosine activation in lung cancer and further affects tumor metastasis. However, ROS is not a specific ligand for EGFR signaling. A recent study reported a novel mechanism whereby angiotensin II causes chronic transactivation of EGFR through ROS activation of Src, which then phosphorylates EGFR at tyrosine 845, leading to sustained EGFR-ERK signaling and phenotypic changes in renal proximal tubule epithelial cells [10]. Our study showed that the expressions of 8-OHdG, p-Src, and p-EGFR in oral carcinoma tissues were significantly higher than those in carcinoma-adjacent tissues (P < 0.05), and statistically significant correlations were observed between 8-OHdG expression and p-Src and p-EGFR expressions (P < 0.05). These results suggest that the ROS-Src-EGFR signaling pathway may play an important role in the development of TSCC.
In recent decades, several studies have suggested that the monoclonal antibody cetuximab has a definite target (EGFR) that is largely expressed in head and neck cancer and is related to tumor aggressiveness. Cetuximab can competitively prevent specific ligands from binding to EGFR, inhibiting its activity; thus, cetuximab has shown treatment efficacy for advanced head and neck cancer. However, the results of searches for clinical or biological features predicting cetuximab sensitivity or resistance have been discouraging [6]. In 2009 Li, et al. reported that the overexpression of EGFR ligands was associated with the movement of the EGFR from the membrane to the nucleus, a process mediated by Src family kinases (SFKs) that caused the loss of the EGFR membrane, increased EGFR signaling in the nucleus, and cetuximab sensitization [21]. The administration of cetuximab in combination with radiotherapy and chemotherapy has shown clear survival improvements within locally advanced and relapsed/metastatic neck and head cancer, respectively [6]. Therefore, blockade of the ROS-Src-EGFR signaling pathway through the combination of cetuximab with another radiotherapy/chemotherapy may represent a unique therapeutic method with significance for clinical treatment. A phase II trial of combined erlotinib (an EGFR inhibitor) and dasatinib (an SFK and multikinase inhibitor) showed the tolerability of the treatment and evidence of antitumor activity in patients with advanced non–small-cell lung cancer [22]. Meanwhile, the use of PEGylated and catalase (ROS inhibitor) inhibited the growth of metastatic malignant tumor cells in vitro experiments [20, 23]. Our study preliminary determined the expression of the ROS-Src-EGFR signaling pathway in TSCC, providing clinical evidence for the use of cetuximab in combination with other chemotherapy drugs for the treatment of oral cancer.
Article Info
Article Type
Research ArticlePublication history
Received: Sun 10, Jun 2018Accepted: Fri 22, Jun 2018
Published: Fri 29, Jun 2018
Copyright
© 2023 Feixin Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2018.02.005
Author Info
Feixin Liang Tao Yu Xiangming Qin
Corresponding Author
Feixin LiangDepartment of Oral and Maxillofacial Surgery,Hospital of Stomatology,Guangxi Medical University,Nanning 530021,Guangxi, China
Figures & Tables
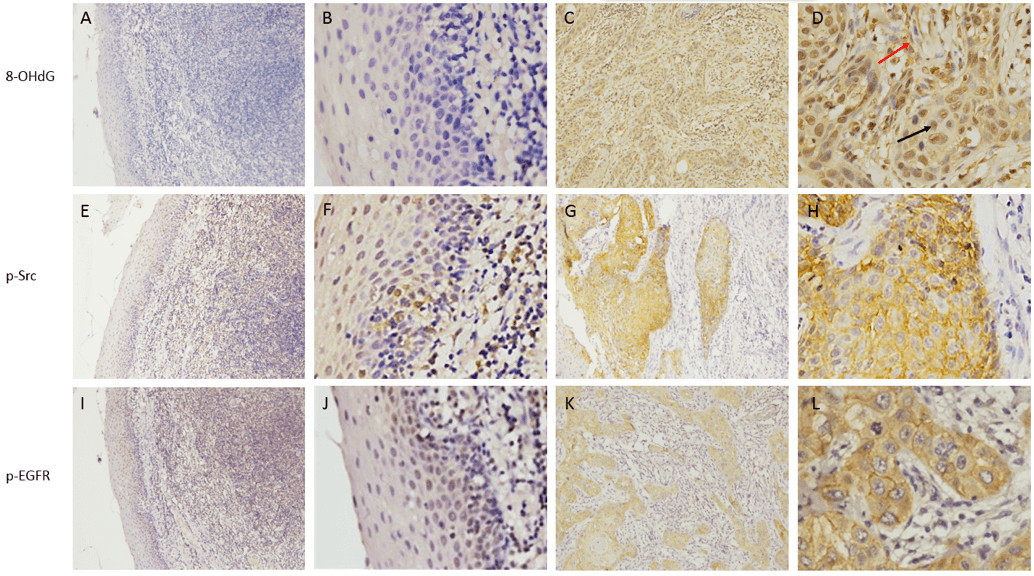
Table 1: 8-OHdG, p-Src, and p-EGFR expression in 40 patients with TSCC
|
|
|
|
8-OHdG |
|
|
p-Src |
|
|
p-EGFR |
|
|||
|
Group |
N |
Negative |
Positive |
P |
Negative |
Positive |
P |
Negative |
Positive |
P |
|||
|
TSCC |
40 |
7 |
33 |
<0.05 |
12 |
28 |
<0.05 |
18 |
22 |
<0.05 |
|||
|
Carcinoma-adjacent tissue |
40 |
33 |
7 |
|
35 |
5 |
|
37 |
3 |
|
|||
Correlations between 8-OHdG expression and p-Src and p-EGFR expression. The expression of 8-OHdG was positively correlated with that of p-Src in both samples (r = 0.387, P < 0.05) and was also positively correlated with p-EGFR expression (r = 0.329, P < 0.05) (Spearman rank correlation test, Table 2).
Table 2: Relationship between 8-OHdG expression and p-Src and p-EGFR expressions
|
|
p-Src |
p-EGFR |
||||||||||
|
8-OHdG |
– |
+ |
++ |
+++ |
R |
P |
– |
+ |
++ |
+++ |
R |
P |
|
— |
2 |
2 |
0 |
0 |
0.387 |
0.011 |
4 |
0 |
0 |
0 |
0.329 |
0.038 |
|
+ |
2 |
0 |
1 |
0 |
|
|
2 |
0 |
1 |
0 |
|
|
|
++ |
1 |
1 |
7 |
5 |
|
|
3 |
2 |
6 |
3 |
|
|
|
+++ |
0 |
4 |
8 |
7 |
|
|
2 |
5 |
11 |
1 |
|
|
References
1. Tsushima N, Sakashita T, Homma A, Hatakeyama H, Kano, et al. (2016) The role of prophylactic neck dissection and tumor thickness evaluation for patients with cN0 tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol 273: 3987-3992. [Crossref]
2. Mengzhu Guo, Yun Mu, Dahai Yu, Jing Li, Fengqiang Chen, et al. (2018) Comparison of the expression of TGF-beta1, E-cadherin, N-cadherin, TP53, RB1CC1 and HIF-1alpha in oral squamous cell carcinoma and lymph node metastases of humans and mice. Oncol Lett 15:1639-1645. [Crossref]
3. Gao L, Yu L, Li CM, Li Y, Jia BL, et al. (2016) Karyopherin α2 induces apoptosis in tongue squamous cell carcinoma CAL-27 cells through the p53 pathway. Oncol Rep 35: 3357-3362. [Crossref]
4. Qian M, Qian D, Jing H, Li Y, Ma C, et al. (2014) Combined cetuximab and celecoxib treatment exhibits a synergistic anticancer effect on human oral squamous cell carcinoma in vitro and in vivo. Oncol Rep. [Crossref]
5. Y Y (2001) The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 37: S3-S8. [Crossref]
6. Numico G, Franco P, Cristofano A, Migliaccio F, Spinazzé S, et al. (2013) Is the combination of Cetuximab with chemo-radiotherapy regimens worthwhile in the treatment of locally advanced head and neck cancer? A review of current evidence. Crit Rev Oncol Hematol 85: 112-120. [Crossref]
7. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, et al. (2009) Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360: 563-72. [Crossref]
8. Schliess F, Reissmann R, Reinehr R, vom Dahl S, Häussinger D (2004) Involvement of integrins and Src in insulin signaling toward autophagic proteolysis in rat liver. J Biol Chem 279: 21294-21301. [Crossref]
9. Qiao L, Studer E, Leach K, McKinstry R, Gupta S, et al. (2001) Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell 12: 2629-2645. [Crossref]
10. Jianchun Chen, Jian-Kang Chen, Raymond C Harris (2012) Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol 32: 981-991. [Crossref]
11. Kimura C, Watanabe K, Iwasaki A, Mori T, Matsushita H, et al. (2013) The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J Matern Fetal Neonatal Med 26: 491-496. [Crossref]
12. Millena Prata Jammal, Allison Araújo Da Silva, Agrimaldo Martins Filho, Eliângela De Castro Côbo, Sheila Jorge Adad, et al. 2015 Immunohistochemical staining of tumor necrosis factor-alpha and interleukin-10 in benign and malignant ovarian neoplasms. Oncol Lett 9: 979-983. [Crossref]
13. Nishikawa M (2008) Reactive oxygen species in tumor metastasis. Cancer Lett 266: 53-59. [Crossref]
14. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49: 1603-1616. [Crossref]
15. Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM (2007) Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer 109: 54-59. [Crossref]
16. Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, et al. (2003) Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res 63: 6478-6487. [Crossref]
17. Heimdal JH, Aarstad HJ, Olofsson J (2000) Peripheral blood T-lymphocyte and monocyte function and survival in patients with head and neck carcinoma. Laryngoscope 110 (3 Pt 1): 402-407. [Crossref]
18. Halliwell B (2007) Oxidative stress and cancer: have we moved forward? Biochem J, 401: 1-11. [Crossref]
19. Grandis J R, J TD (1993) Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 53: 3579-3584. [Crossref]
20. Hyoudou K, Nishikawa M, Kobayashi Y, Umeyama Y, Yamashita F, et al. (2006) PEGylated catalase prevents metastatic tumor growth aggravated by tumor removal. Free Radic Biol Med 41: 1449-1458. [Crossref]
21. Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL (2009) Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene 28: 3801-3813. [Crossref]
22. Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, et al. (2010) Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol 28: 1387-1394. [Crossref]
23. Nishikawa M, Hashida M, Takakura Y (2009) Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Advanced Drug Delivery Reviews 61: 319-326.
