Early Results of a Novel Treatment Technique with Autologous Blood for Chronic Lateral Epicondylitis
A B S T R A C T
Background: Chronic lateral epicondylitis can be a severe disabling condition. There is still lack of consensus on best treatment, as no single intervention has been proven to be superior regarding pain relief and improvement of function. Due to the self-limiting nature of this elbow condition, we are looking for a fast and safe treatment method to break through this pattern of pain and loss of elbow function. Autologous blood injection therapy by means of an automatic injection system, can be a promising new treatment option for this group of patients suffering from chronic lateral epicondylitis. In this study, we evaluated the short-term results of autologous blood injection therapy in a standardized way by using an automatic injection system (=ITEC device) for the treatment of chronic lateral epicondylitis.
Methods: A total of 141 patients with chronic lateral epicondylitis (88 female, 53 male) were enrolled in this clinical treatment evaluation being treated with the ITEC device. The mean age of the patients was 50.0 years (19 years-73 years). Numeric rating scale (NRS) and a patient reported outcome measurement tool (Oxford Elbow Score (OES)) were measured at baseline, six weeks and three months follow-up.
Results: Pain (NRS, OES) and elbow function / quality of life (OES) were significantly improved within 6 weeks after ITEC treatment. This improvement in NRS and OES sustained during the 3 months follow-up period.
Conclusion: Autologous blood injection therapy by means of a new automatic injection system (ITEC device) is a safe and effective treatment method for patients with chronic lateral epicondylitis. More research is necessary to see if this effectiveness sustains in the long-term follow-up.
Keywords
ITEC, autologous blood, lateral epicondylitis, injection technique, chronic tennis elbow
Introduction
Lateral epicondylitis is one of the most prevalent diseases of the elbow. In the general Dutch population, 7 in 1000 patients are diagnosed each year. It mainly effects people in their working age, with a peak between 40 and 50 years of age, without gender-based differences [1]. The most common presentation involves pain over the lateral epicondyle of the humerus in combination with loss of elbow function. This tendinopathy is histologically characterized by degenerative changes that are related to repetitive stress and overuse injury, typically of the extensor carpi radialis brevis tendon. Consequently, these repeated microtraumas cause partial or full rupture of the extensor, which originates at the lateral epicondyle of the humerus. With the decrease of collagen accumulation and increase of neovascularization, angiofibroblastic hyperplasia develops [2, 3]. Until now, there is a lack of consensus on which is the best treatment option for lateral epicondylitis and the choice of treatment is mainly based on personal preferences of the treating physician [4]. Several conservative treatment options have been described, of which the most important include watchful waiting avoiding physical overload, possibly combined with a course of nonsteroidal inflammatory drugs; dry needling; functional bracing; manual treatment and local injection therapies with autologous blood, platelet-rich plasma, steroids or botulin. None of these above-mentioned treatments have shown superior results, especially when pain is persistent and severe [5-7].
Generally, lateral epicondylitis is a self-limiting condition that will resolve in approximately 80% of cases within a period of six to twelve months [8, 9]. However, in 25% of the cases, the disease does not resolve within this period of time and subsequently can lead to chronic therapy resistant discomfort and evident functional disability. Consequently, this leads to high costs due to productivity loss and health care use [10-12]. It is therefore important to look at possible treatment options that could relieve patients’ symptoms in a timely and unambiguous manner. More recently, biological treatment options, which include autologous blood and platelet-rich plasma, are being popular. These treatment modalities are based on the above-described pathophysiological mechanism involved in lateral epicondylitis. It is hypothesized that autologous blood preparations may speed up the natural healing process by initiating an inflammatory process around the tendon. This would lead to cellular and humoral mediators that could increase de rate of tendon repair. Subsequently, it allows for the delivery of nutrients and high concentration of growth factors. These are believed to stimulate the body’s own healing mechanism by increasing new collagen formation and neovascularization [13, 14]. Some previous studies have shown favourable results regarding relief of pain and functional improvement by using autologous blood in treating chronic lateral epicondylitis [15, 16].
In general, results remain omnifarious though and current studies correlate poorly. Not least, because of a great diversity in execution of injection techniques. In most cases, the injections are performed manually and blindly, with often no ultrasound guidance. Also, the amount and depth of the perforation is often not defined [17, 18]. Therefore, comparison of results remains questionable. In 2017, a new automatic injection device (ITEC device) which involves autologous blood has emerged as a promising treatment option in patients with chronic lateral epicondylitis. This device subsequently provides for a standardized injection technique which rules out manual differences [18]. The primary aim of this study is to evaluate the short- term effectiveness of ITEC within 3 months of treatment. When statements on the effectiveness of ITEC can be made, this device can provide in a more universal method of treatment, and hopefully, unnecessary treatments can be avoided in the future.
Materials and Methods
In the period 2017-2019, hundred and forty-three patients presenting with chronic lateral epicondylitis in our outpatient clinic of the department of orthopaedic surgery in a medium-sized cooperating top clinical training hospital in Amersfoort, the Netherlands, were included in this clinical treatment evaluation. Our local medical review committee approved the (case no: TWO 20-034-ITEC). Chronic lateral epicondylitis was defined as pain over the lateral elbow epicondyle with direct palpation and resisted wrist extension, with symptoms being present for more than 6 months nonresponding to conservative treatment, or symptoms increasing from 3 months onwards. To rule out other medical conditions which can be accompanied by elbow pain (i.e., calcifications, arthrosis, tumor), X-ray and diagnostic ultrasonography of the elbow was obtained prior to treatment. All subjects had failed previous conservative treatment. Patients with coexisting pathology (rheumatoid arthritis, cervical radiculitis, carpal tunnel syndrome) were excluded from this clinical treatment evaluation. A patient information form including a link to the website (Link 1) was handed out to all patients prior to treatment. If the patient agreed to participate to the new treatment method, they were included to the follow-up.
I ITEC Device Technique
This device provides a standardized method for treating lateral epicondylitis. By making use of this ultrasound guiding device, the exact location can be found on the affected elbow and perforation of the tendon to the proper depth with 12 needles (3x4 design of ITEC-injection disposables) is being executed in a reproducible and controlled way. The skin is locally anaesthetised by means of a local field block technique. Hereafter, diagnostic ultrasound of the affected elbow is being performed to check for local pathologies and depth of the common extensor tendon, followed by signing of the anatomic landmarks. Subsequently, autologous venous blood is drawn from the contralateral upper limb. Then, with help of an automatic percutaneous injection system (=ITEC device), 12 holes are accurately pricked in the affected tendon and simultaneously two mL of venous blood is injected starting proximally and going along the supracondylar ridge up to the under surface of ECR. After the procedure, patients were advised to avoid strenuous activities for the first two weeks after which they could resume their normal activities. The procedure was done by two of the authors (HS and TB).
II Primary Outcome
The main outcome was the change in the perception of pain, which was measured by using the Numeric Rating Scale (NRS). This 11-point numeric ranges from “0” representing one pain extreme (e.g., “no pain”) to “10” representing the other pain extreme (e.g., “pain as bad as you can imagine” or “worst pain imaginable”). Patients were asked to score their degree of pain at baseline (before treatment) and at six weeks and three months after treatment.
III Secondary Outcome
The secondary outcome measure includes elbow function and quality of life, measured with the Oxford Elbow score. The Oxford Elbow Score (OES) is a validated 12-item patient-related outcome measurement tool specifically designed and developed for assessing outcomes of elbow surgery. It is used to quantify a patients’ quality of life with regard to having an elbow disorder. Herewith, a higher score corresponds with a higher quality of life. Assessments were made at baseline, six weeks and three months after treatment.
IV Demographic Variables
Descriptive data of all participants were collected before treatment regarding age, sex and dominant arm.
V Statistical Analysis
To evaluate whether the distribution of the continuous variables was normal, the Kolmogorov-Smirnov test was used. Wilcoxon Signed Rank test was used for multiple comparisons. Patients with missing data at any point of follow-up were not taken into analysis. Variables were presented as the mean ± standard error. A p-value <0,05 was considered significant. Analyses were performed using IBM SPSS statistics for windows, version 24.0.
Results
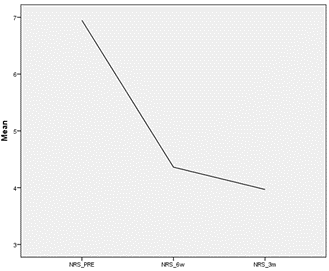
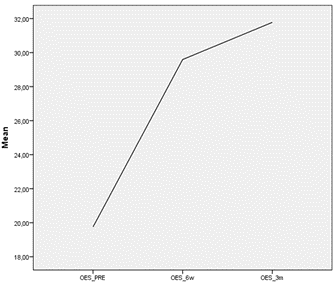
The study included 141 patients (88 females and 53 males) clinically diagnosed with chronic lateral epicondylitis, who all failed to respond to previous conservative therapy. 136 patients completed the 6-week follow-up visit as scheduled. 33 patients participated in the visit conducted 3 months after ITEC treatment. The mean age of the group was 50.0 years (±9.0). In 67% of cases, the dominant side was affected. The right side was more frequently involved (61%). The mean baseline NRS score was 6.9 (±0.1) and showed significant reduction of 36% within 6 weeks after treatment (p<0.001). The score further improved at 3 months after treatment, with a mean reduction of 56% from baseline (p<0.001) (Figure 1). Patient reported functional health and well-being, measured with the OES, also showed significant improvement throughout the follow-up period, increasing from a mean score of 19.8 (±0.7) before treatment to a mean score of 29.4 (±0.8) at six weeks- and 32.5 (±2.0) at 3 months follow-up (p>0.001) (Figure 2). There were no serious adverse events and no reports of infection after ITEC treatment. The most common adverse event was minor swelling and/or pain at the site of injection, which typically resolved within a few days of treatment.
Figure 1: Mean NRS outcome pre, 6 weeks and 3 months post ITEC-treatment.
Figure 2: Mean OES outcome pre, 6 weeks and 3 months post ITEC-treatment.
Discussion
Previous studies have shown favourable results regarding relief of pain and functional improvement by using autologous blood in treating chronic lateral epicondylitis [15, 16]. For instance, Peerbooms et al. showed that 73% of patients treated with autologous blood experienced a ≥25% reduction in pain scores within 1 year [19]. However, comparing this result with the natural course of lateral epicondylitis, which is in general a self-limiting condition resolving in approximately 80% of cases within a period of six to twelve months, then this is somewhat disappointing [9]. Looking in detail at more current studies evaluating the effectiveness of biological injectables for lateral epicondylitis, we see a poor correlation, often due to the lack of a standardized treatment algorithm. Presumably also due to the lack of a standardized injectable technique executed in a reproducible and controlled way. It seems like current choice and manner of treatment for lateral epicondylitis is mainly based on personal preferences of the treating physician [17, 18]. Chronic lateral epicondylitis can lead to evident functional disability, which consequently, leads to high costs due to productivity loss and health care use [11]. It is therefore important to establish treatment options that could relieve patients’ symptoms in a safe and timely outpatient manner. The relatively short follow-up period limits predictions on sustainability of ITEC treatment. However, again looking at the natural course of lateral epicondylitis short-term effect benefits of treatment in terms of pain, productivity loss, quality of life and disability, become particularly important.
Dworkin et al. provided estimates of changes in NRS scores that correspond to clinically significant improvement in patients suffering from chronic pain. It was shown that a 10% reduction in NRS score was considered the minimum amount to be clinically significant [20]. Our results show a 36% reduction in NRS score at six weeks after treatment, which improved further to 56% at three months follow-up. Also, patient-reported quality of live and elbow functioning, measured with the Oxford Elbow score, corroborated with the reduction in pain scores and where significantly increased at both six weeks- and three months of follow-up. No serious adverse events were seen, and no one withdrew from participation in this study. The most common adverse event reported was transient post injection pain. This finding corresponds with results of other studies that also showed temporary signs of post injection pain in most patients, often resolving within 2 days [21].
Although our prospective cohort study showed encouraging clinical results, with both functional improvement and significant reduction in pain possibly partly established because of the standardized automatic injection method which can be more exact in location and perforation depth with 12 needles (instead of traditionally 1 needle), executed in a simple, reproducible and controlled way. The small number of patients especially at 3 months follow-up and absence of a control group were limitations of our study. So, more research is necessary to compare this standardized injection technique with other common treatment modalities.
Conclusion
Chronic lateral epicondylitis is a severe disabling condition. No single intervention has been proven to be superior regarding pain relief and improvement of function. It was hypothesized that autologous blood injection therapy by means of an automatic injection system (=ITEC) can be a promising new treatment option for this group of patients suffering from chronic lateral epicondylitis. The results in our study have shown that autologous blood injection therapy by means of the ITEC device is a safe and effective treatment method for patients with chronic lateral epicondylitis in the short-term. More research is necessary to see if this effectiveness sustains in the long-term follow-up.
Declaration
Local approval committee number: TWO 20-034-ITEC. Level of evidence: Level IV.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 21, Jun 2021Accepted: Fri 17, Sep 2021
Published: Tue 28, Sep 2021
Copyright
© 2023 Michelle Coopmans. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.09.10
Author Info
Michelle Coopmans Heleen Sonneveld Thomas Berendes
Corresponding Author
Michelle CoopmansDepartment of Orthopaedic Surgery, Meander Medical Centre, Amersfoort, Netherlands
Figures & Tables
References
1.
Chiavaras MM, Jacobson
JA, Carlos R, Maida E, Bentley T et al. (2014) IMpact of Platelet Rich plasma OVer alternative therapies in
patients with lateral Epicondylitis (IMPROVE): protocol for a multicenter
randomized controlled study: a multicenter, randomized trial comparing
autologous platelet-rich plasma, autologous whole blood, dry needle tendon
fenestration, and physical therapy exercises alone on pain and quality of life
in patients with lateral epicondylitis. Acad Radiol 21: 1144-1155. [Crossref]
2.
Cook JL, Purdam CR
(2009) Is tendon pathology a continuum? A pathology model to explain the
clinical presentation of load-induced tendinopathy. Br J Sports Med 43:
409-416. [Crossref]
3.
Bazancir Z, Fırat T
(2019) A potential factor in the pathophysiology of lateral epicondylitis: The
long sarcomere length of the extensor carpi radialis brevis muscle and
implications for physiotherapy. Med Hypotheses 130: 109278. [Crossref]
4.
Coombes BK, Bisset
L, Vicenzino B (2015) Management of Lateral Elbow Tendinopathy: One Size Does
Not Fit All. J Orthop Sports Phys Ther 45: 938-949. [Crossref]
5.
Placzek R, Drescher
W, Deuretzbacher G, Hempfing A, Meiss AL (2007) Treatment of chronic radial
epicondylitis with botulinum toxin A. A double-blind, placebo-controlled,
randomized multicenter study. J Bone Joint Surg Am 89: 255-260. [Crossref]
6.
Krogh TP, Bartels
EM, Ellingsen T, Stengaard Pedersen K, Buchbinder R et al. (2013) Comparative
effectiveness of injection therapies in lateral epicondylitis: a systematic
review and network meta-analysis of randomized controlled trials. Am J
Sports Med 41: 1435-1446. [Crossref]
7.
Radwan YA, ElSobhi
G, Badawy WS, Reda A, Khalid S (2008) Resistant tennis elbow: shock-wave
therapy versus percutaneous tenotomy. Int Orthop 32: 671-677. [Crossref]
8.
Matache BA,
Berdusco R, Momoli F, Lapner PLC, Pollock JW (2016) A randomized, double-blind
sham-controlled trial on the efficacy of arthroscopic tennis elbow release for
the management of chronic lateral epicondylitis. BMC Musculoskelet Disord
17: 239. [Crossref]
9.
Vaquero Picado A,
Barco R, Antuña SA (2017) Lateral epicondylitis of the elbow. EFORT Open Rev
1: 391-397. [Crossref]
10.
Walker Bone K,
Palmer KT, Reading I, Coggon D, Cooper C (2012) Occupation and epicondylitis: a
population-based study. Rheumatology (Oxford) 51: 305-310. [Crossref]
11.
Shiri R, Viikari
Juntura E, Varonen H, Heliövaara M (2006) Prevalence and determinants of
lateral and medial epicondylitis: a population study. Am J Epidemiol
164: 1065-1074. [Crossref]
12.
Kahlenberg CA,
Knesek M, Terry MA (2015) New Developments in the Use of Biologics and Other
Modalities in the Management of Lateral Epicondylitis. Biomed Res Int
2015: 439309. [Crossref]
13.
Arirachakaran A,
Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W et al.
(2016) Platelet-rich plasma versus autologous blood versus steroid injection in
lateral epicondylitis: systematic review and network meta-analysis. J Orthop
Traumatol 17: 101-112. [Crossref]
14.
Creaney L, Wallace
A, Curtis M, Connell D (2011) Growth factor-based therapies provide additional
benefit beyond physical therapy in resistant elbow tendinopathy: a prospective,
single-blind, randomised trial of autologous blood injections versus
platelet-rich plasma injections. Br J Sports Med 45: 966-971. [Crossref]
15.
Jindal N, Gaury Y,
Banshiwal RC, Lamoria R, Bachhal V (2013) Comparison of short term results of
single injection of autologous blood and steroid injection in tennis elbow: a
prospective study. J Orthop Surg Res 8: 10. [Crossref]
16.
de Vos RJ, van
Veldhoven PLJ, Moen MH, Weir A, Tol JL et al. (2010) Autologous growth factor
injections in chronic tendinopathy: a systematic review. Br Med Bull 95:
63-77. [Crossref]
17.
Lai WC, Erickson
BJ, Mlynarek RA, Wang D (2018) Chronic lateral epicondylitis: challenges and
solutions. Open Access J Sports Med 9: 243-251. [Crossref]
18.
Keijsers R, Kuijer
PPFM, Koenraadt KLM, van den Bekerom MPJ, Gerritsma Bleeker CLE et al. (2019)
Effectiveness of standardized ultrasound guided percutaneous treatment of
lateral epicondylitis with application of autologous blood, dextrose or
perforation only on pain: a study protocol for a multi-center, blinded, randomized
controlled trial with a 1 year follow up. BMC Musculoskelet Disord 20:
351. [Crossref]
19.
Peerbooms JC,
Sluimer J, Bruijn DJ, Gosens T (2010) Positive effect of an autologous platelet
concentrate in lateral epicondylitis in a double-blind randomized controlled
trial: platelet-rich plasma versus corticosteroid injection with a 1-year
follow-up. Am J Sports Med 38: 255-262. [Crossref]
20. Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS et al. (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9: 105-121. [Crossref]
21. Dojode CM (2012) A randomised control trial to evaluate the efficacy of autologous blood injection versus local corticosteroid injection for treatment of lateral epicondylitis. Bone Joint Res 1: 192-197. [Crossref]
