Directionality of Chemical Reaction and Spontaneity of Biological Process in the Context of Entropy
A B S T R A C T
Chemical and biochemical reactions are carried out either to generate energy or to produce useful macromolecules. Entropy is a well-applied concept in many fields, including physics, chemistry, biology, and medicine. Various perspectives have been used to describe the concept, creating confusion and misconceptions. In chemical and biochemical reactions, entropy plays a significant role in the directionality and spontaneity of the reactions. Potential energy can be used to better understand the concept of entropy. Potential energy represents order, while entropy represents disorder; therefore, they are inversely proportional and intimately linked. Molecules with high potential usually have rich sets of functions and information, which is due to the enrichment of their constitutions, configurations, and conformations. In molecules with low potential, there are greater vibrational, rotational, and translational motions associated with decreased order in their constitution, configuration, and conformation. Distribution of electronic charge changes in macromolecules over time, increasing the rotation of side-chain residues and thus increasing entropy and affecting potential in terms of structure, function, and information. Entropy can thus be defined as a state of spontaneous change, bound to time and constantly increasing, which causes structural changes in the form of constitution, configuration, and conformation, and functional changes in the form of the ability to do work as well as informational changes in the form of the transmission of commands.
Keywords
Entropy, potential energy, structure, function, information, halalopathy
Introduction
Entropy is one of the terms used in textbooks and literature in a variety of descriptions and interpretations that often confuse students and teachers [1]. In general, it is characterized by disorder, randomness, irregularity, high probability, and uncertainty [2]. Other interpretations describe it as dispersed or suppressed energy released in the form of heat [3]. Some consider entropy to be a wasteful form of energy and therefore treat it as energy of little value [4]. Entropy, on the other hand, is described as a state function related to the number of microstates of a system (the number of possible constitutions, configurations, and conformations in which the system can be arranged) [5]. Qualitatively, entropy is a measure of the possible arrangement of atoms, ions, or molecules and their dispersion or distribution of energy in a substance [6]. Quantitively, entropy is a thermodynamic quantity commonly used to describe the course of a process, i.e., whether it is spontaneous and can proceed in a particular direction, or if it is non-spontaneous and can proceed in the opposite direction [7]. The term entropy provides useful information for understanding phase changes, energy forms, temperature changes, or pressure changes, as well as explaining passive or spontaneous processes.
To understand the concept of entropy, it is important to recognize what circumstances lead to an increase or decrease in entropy and what the pre-entropy phase is, and how they are connected. It is obvious that increasing temperature, decreasing pressure, and the movement of particles from an area of high concentration to an area of low concentration, increase entropy. Halalopathy, a new concept in medicine, uses entropy and potential energy as key elements for prevention and healing [8]. Entropy represents disorder, and the phase preceding entropy is the phase of order represented by potential energy; consequently, the concepts of entropy and potential energy are closely related and inversely proportional. Therefore, it is complicated to understand the concept of entropy without including the concept of potential energy in the description. Since they are closely related, understanding both concepts together will open a new window for energy management in terms of structure, function, and information [9, 10]. Accordingly, the use of potential energy and entropy concepts in medicine could lead to a new orientation for a better understanding of biological processes and thus improve the prevention and treatment of diseases.
Entropy in Chemical reactions
Chemical reactions are carried out either to generate energy or to produce useful products. Predicting the directionality of reactions remains one of the greatest challenges in organic chemistry and a prerequisite for effective synthetic design. Many chemical reactions are reversible and yet proceed in a direction referred to as spontaneous. This raises an obvious question: what makes a reaction spontaneous and what drives the reaction in one direction and not the other? In chemical reactions, it is difficult to predict the directionality and spontaneity of the reaction by looking only at the changes in enthalpy (∆H), which represents the changes in heat as the substance is formed. In general, chemical reactions proceed in the direction that leads to an increase in the disorder of the system and consequently to an increase in entropy (∆S). The reaction tendency is thus determined by two driving forces, namely the changes in enthalpy and entropy that occur during the reaction [11, 12]. Therefore, the Gibbs free energy was introduced to represent the relationship between entropy and enthalpy and to provide information useful for predicting the directionality and spontaneity of a reaction [13]. According to the Gibbs free energy (∆G = ∆H - T∆S), the following points are relevant for determining the directionality of the reaction:
i. A negative ∆H and a positive ∆S cause a reaction to being spontaneous and exothermic at all temperatures, vice versa, then non-spontaneous and endothermic.
ii. If ∆S is positive, a higher temperature (T) makes a reaction more spontaneous. If ∆S is negative, a higher T makes a reaction less spontaneous.
According to the first law of thermodynamics, the total amount of energy at the beginning of a chemical reaction must be equal to the total amount of energy at the end of the reaction [14]. In general, energy represents the ability of an object to do work, exert a function, cause a change in a process, or provide heat. At the molecular level, chemical potential energy is stored in the constitution, configuration, and conformation of the molecule and has the potential to do work when energy is released [15]. Molecules with high potential are usually rich in functions and information, which is mainly due to an enrichment of their constitution, configuration, and conformation [16]. Molecules with low potential lose function and information due to less order in their constitution, configuration, and conformation associated with increased vibrational, rotational, and translational motions. An exothermic reaction is possible when molecules with a high potential structure are converted into molecules with a lower potential structure. The lost potential is converted into entropy, which manifests as heat emission and temperature increase. The new molecule has a smaller size (constitution), less spatial orientation (configuration), and more free rotation (conformation) with increased vibration, rotation, and translational motions [17].
The total energy of a system is referred to as the internal energy and is equivalent to the work (potential energy) and heat (entropy); consequently, the potential energy decreases when the entropy increases and vice versa. According to the second law of thermodynamics, under any spontaneous reaction or process, the change in total entropy of a system and its surroundings is always positive. Indeed, the increase in entropy plays an important role in the irreversibility of many reactions and natural processes. The entropy change is evident in the following relationships:
i. If reactions take place in solution or in heterogeneous systems, the generation of gas contributes significantly to the overall entropy change of the reaction.
ii. If all reactants and products are gases, the entropy change is positive in the direction of the reaction that produces more gas molecules overall.
iii. Entropy also increases during the transition from a state with lower energy to a state with higher energy (solid → liquid → gaseous).
iv. The conversion of macromolecules into micro-molecules is usually associated with an increase in entropy.
v. At the macromolecular level, the transition from a native state to → denatured state → individual building blocks (monomers) increases entropy. In the denatured state, the potential in the form of the conformation is lost, while at the level of the monomers, the overall potential in the form of the constitution, configuration, and conformation is reduced.
It is important to emphasize that the rate of a reaction is independent of its spontaneity. A reaction can be spontaneous, but the rate of transformation is very slow, such as the transformation of diamond into graphite, which is a spontaneous but very slow process [18].
Entropy in Living Systems
It is common to study entropy at different temperatures and/or pressures. However, to simplify the concept and to gain a deeper insight into entropy, it is useful to study the changes in reactions, phases, diffusion, and energy forms at constant temperature and pressure. This state is commonly found in living systems such as humans, where temperature and pressure are approximately constant under non-disease conditions. The disease has always been referred to as a disorder, and the disorder can normally occur in the human body in two states, a physical and non-physical disorder. A physical disorder is a functional disorder, such as a dysfunction of the human organs (heart, lungs, kidneys, etc.), while a non-physical disorder is an informational disorder, such as a mental illness [19].
Entropy represents disorder, while potential energy represents order; therefore, the two concepts are closely related and inversely proportional [20]. To better understand the concept of entropy, especially in the context of the human body, it is important to consider entropy from the perspective of potential energy and its connection to metabolic processes. In the human body, biochemical reactions are carried out either to generate energy or to produce useful macromolecules; thus catabolic and anabolic reactions are essential metabolic processes required to generate ATP and build highly vital molecules [21]. The key factors that determine the direction of these reactions can be well understood by looking at the two pathways from a molecular perspective:
I Catabolic Reaction
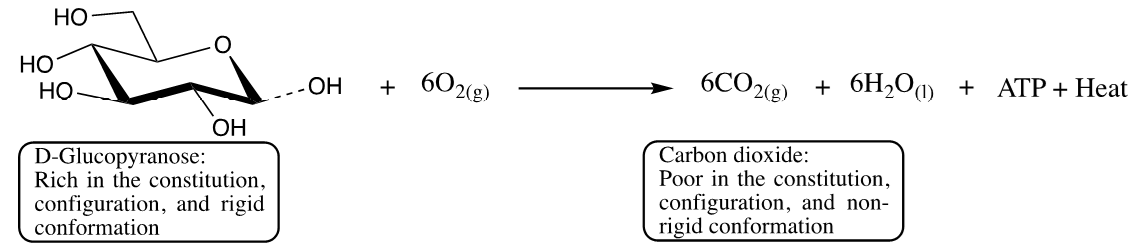
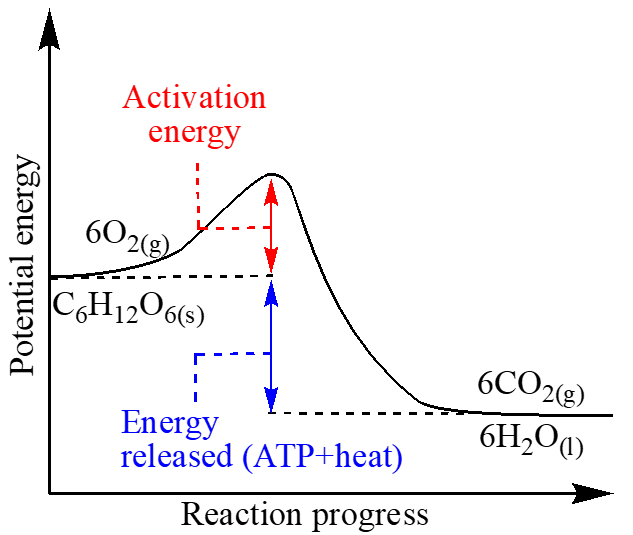
In catabolic reactions, macromolecules such as carbohydrates, fats, DNA, and proteins from the ingested food are broken down into their building blocks. The catabolic reaction requires only a minimal amount of energy to initiate the reaction, but throughout the catabolic process, an enormous amount of energy is released in the form of ATP [22]. The catabolic reaction, therefore, occurs spontaneously and independently of the availability of energy, and the driving force that triggers the catabolic reaction is mainly due to the increase in entropy (anti-potential energy), which in turn is converted into heat to maintain body temperature. Entropy increases when highly ordered and well-defined macromolecules break down the molecular constitution into less defined and less ordered micromolecules. When glucose is oxidized (Figure 1) by the catabolic process, the total potential energy stored in the constitution, configuration, and conformation of the glucose structure is reduced [23-34]. The glucopyranose constitution is degraded, the chiral carbons and their relative configuration are lost, the rigidity of the chair conformation is dissipated, and the overall disorder in terms of function and information is reduced [3,8]. The oxidation reaction is exothermic (Figure 2) in terms of enthalpy change (bond breaking and bond formation), potential energy change (structural connectivity of reactant and product), and entropy change (rotational and collisional behaviour of the reactants and the product).
Figure 1: Catabolic oxidation of glucose in the presence of oxygen.
Figure 2: Energy diagram for catabolic/spontaneous/exothermic reaction of glucose.
The potential energy change is stored as ATP and the entropy change is released in the form of heat. During the oxidation of glucose, entropy increases in two places: first, the number of moles of the products (6 moles of CO2 and 6 moles of H2O) increases compared to the reactants (one mole of glucose and 6 moles of O2), and second, is due to changes of state as one mole of glucose in the solid state is converted into 6 moles of CO2 in the gaseous state, while 6 moles of oxygen in the gaseous state are converted into 6 moles of water in the liquid state. Overall, the potential energy of glucose decreases while the entropy increases, whereas the potential energy of oxygen slightly increases and the entropy slightly decreases, so that the overall reaction: quantitatively, the potential energy decreases and the entropy or anti-potential energy increases, allowing the reaction to proceed spontaneously. It is important to point out here that the potential energy and entropy of a complex molecule like glucose are higher than that of a simple molecule like oxygen; thus, it is inequitable to compare the entropy of glucose with that of oxygen without considering the potential energy of both molecules.
II Anabolic Reaction
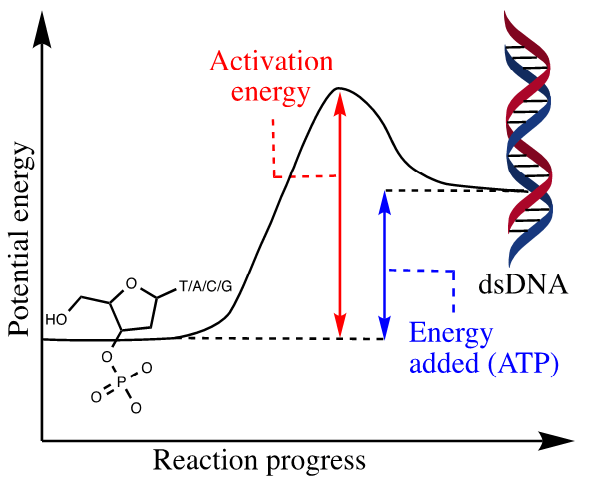
The anabolic reaction or biosynthetic reaction is the reverse process of the catabolic reaction in which small molecules or building blocks of low potential are assembled to form a well-defined macromolecule such as proteins, lipids, and nucleic acids with a more precise constitution, configuration, and conformation and consequently with enriched functional and informational macromolecules. The anabolic reaction uses ATP, which comes from catabolic reactions, as the energy source; therefore, the anabolic process is not spontaneous and depends on the availability of potential energy [25]. Anabolic reactions, for example, link four different deoxynucleotides to form new DNA strands (Figure 3). This process requires potential energy in the form of ATP molecules, which are normally provided by catabolic reactions. The insufficient availability of energy (ATP) slows down the anabolic process and can result in the inability to continue the growth or synthesis of vital macromolecules [26]. Consequently, the entropy that drives the catabolic process acts as a source of anti-potential energy and manifests itself as spontaneous or involuntarily acquired energy and can proceed independently of the availability of potential energy, whereas the anabolic process is driven by the availability of potential energy (ATP). Anti-entropy or potential energy can manifest itself as non-spontaneous or voluntarily acquired energy.
Figure 3: Energy diagram for anabolic/non-spontaneous/endothermic reaction of deoxynucleotides monomers.
Any disorder, whether in terms of structure, function, or information, can be triggered by particles, atoms, ions, molecules, or waves [27]. Micromolecules are usually less well-defined and more flexible in their movement, thus exist in high entropy. In other words, if micromolecules are not utilized as building blocks for anabolic macromolecular synthesis, they must be excreted from the body. In general, as micromolecules have a lower potential and function, and because they are less defined, their free movement increases the collision with other molecules and or with the walls of the container. These collisions generate heat, which ultimately raises the temperature of the closed system, increases the kinetic energy, and causes other macromolecules to vibrate violently and rapidly, weakening the strong bonds and breaking the weak bonds [28]. The anabolic process counteracts entropy; therefore, it is a challenge to keep the anabolic process active, especially in older people. The methods for enriching potential energy have been described extensively in the literature [29, 30].
Macromolecules in Terms of Potential and Entropy
Macromolecules play an essential role in living systems and have a high level of potential energy, thus more effective function and significant information compared to a highly entropic building block, monomers [31, 32]. The constitution, configuration, and conformation of the native state of the macromolecule are rich in potential energy. The restricted conformation and low atomic rotation are the results of cooperative non-covalent intramolecular forces caused by hydrogen bonding and hydrophobic interactions, which compensate for the low entropy and sustain the constitution and configuration of the macromolecules [33]. Cells are constantly exposed to both exogenous and endogenous stressors that threaten the integrity and stability of the genome and lead to an accumulation of disorders, causing entropy to increase [34]. This generates heat, which leads to the denaturation of the macromolecule. Heat has the ability to increase the vibrational, rotational, and translational motions of the macromolecule, thereby interrupting the cooperative effect and decreasing the strength of intramolecular forces such as hydrogen bonds and nonpolar hydrophobic interactions. As a result, there is a decrease in conformational constraint and an increase in atomic rotation of the macromolecular residues, ultimately a decrease in potential energy [35]. Consequently, the self-assembled, rigid, and native structure is distorted; meanwhile, complexity, facticity, and sustainability are lost, water molecules around the macromolecule are rearranged, the number of conformations, rotations, microstates, and phases are increased, molecular movements become uncoordinated, scattered in all directions. The denatured macromolecule is therefore, in a state of high entropy and is unable to retain precise information and perform an effective function.
During the aging process, three main defects appear in the human body, a structural defect, a functional defect, and an informational defect [36]. The structural and functional defects manifest in the functional weakness of human organs, while the informational defect manifests itself in memory loss and lack of interest in life. All defects are the result of spontaneous changes that are bound to time and are accompanied by enhanced entropy. Essential values such as human, moral and spiritual values play an important role in the development of potential; therefore, a constant enrichment of positive personality traits is necessary to maintain mental and informational stability.
Discussion
Entropy is probably the most misunderstood thermodynamic property. While temperature, pressure, and volume can be easily measured, entropy cannot be directly and precisely determined. In chemical reactions, entropy has to do with the loss of constitution, configuration, and conformation of the molecular structure and the increase in vibrational, rotational, and translational motions. If a reaction is entropy-driven and exothermic, no product with 100 % potential energy can be generated; part of this potential is lost in the form of entropy, and in the following sequence: conformation → configuration → constitution. Potential energy can be used to generate work, while entropy generates heat and raises the temperature [37, 38]. The energy distribution is evident in the oxidation of glucose to carbon dioxide and water. Energy is released in the form of potential energy and entropy. Potential energy is stored in the form of ATP, which later can be used to perform work such as anabolic reactions, whereas entropy is used as a source of heat to maintain body temperature at 37 °C. When a reaction is driven by potential energy and is endothermic, potential energy is added from an external source to compensate for the decrease in entropy. Anabolic products usually have higher potential energy than reactants and are stored in bonds, spatial orientation, and self-organization, which translate into functions and information. Thus, in the body, the disorder can have three faces: structural, functional, and informational.
The structural disorder is due to changes in the constitution, configuration, and conformation of the molecule, while functional disorder is due to the disordered orientation and electronic distribution of the molecules, while the informational disorder is related to either a genomic disorder or a psychological trait disorder. Errors in the transfer of information from DNA to RNA and then to protein can significantly impair gene function and lead to misfolded and poorly functioning proteins. Misfolded proteins accumulate in neurodegenerative diseases and must be recognized and degraded by the immune system. The reduction of cause-effect information and the loss of compatibility leads to an increase in disorder. Information can be represented by a wave function, i.e., the more matching between conceptual waves and behavioural waves, the more entanglement and thus more potential is generated, while a mismatch leads to a dispersion or suppression of potential energy and ultimately to a loss of entanglement and an increase in entropy.
In biology, it is generally known that structure determines function. A structure-function relationship exists when the individual structural elements of a system are interconnected and assembled into a structural arrangement that enables the system to carry out its activity [39]. The specific function is determined by the specific constitution, configuration, and conformation within the structural molecule. Any change in the molecular structure leads to an accumulation or reduction of potential energy and thus to an altered or impaired function. Protein-ligand complexes are uniquely useful for functional information. A ligand with a well-defined structure, enriched chiral centers, and restricted conformation leads to stronger binding interaction and thus more effective function. Maintaining structure, potential and thus function is a challenge. Entropy arises and accumulates over time, while at the same time potential energy is lost. However, the accumulation of potential energy through appropriate nutrients, exercise, and anabolic thinking would help to slow down the accumulation of entropy and thus slow down the aging process [36].
Information has two sources, endogenous and exogenous, i.e., endogenous information relates to structure and function, while exogenous information is received in either a supportive or destructive form. The brain acts as an information-processing organ, and information flows from the brain to the rest of the body's cells in the form of commands. The endogenous information is stored as genetic and epigenetic information. As long as the structure and sequence of the DNA and the epigenetics are preserved, the information is stored properly, but over time many DNA breaks and mutations occur. After many repairs, the proteins involved in the repair do not revert to the required structure and position, changing the distribution of electronic charge, increasing the rotation of side chain residues, and thus raising entropy and affecting the structure, function, and information. As a result, an entropy-related body is defined as a state of spontaneous change that is bound to time, and constantly increasing, and results in structural changes in the form of constitution, configuration, and conformation; functional changes in the form of the ability to perform actions; and informational changes in the form of the command transmission.
In halalopathy, entropy and potential energy are fundamental elements necessary for understanding all physical objects and natural processes [40-43]. The concept of potential energy is represented by voluntarily acquired or non-spontaneous energy, while entropy is represented by involuntarily acquired or spontaneous energy. Both opposing forces are found in all objects and have been used as a basis for halalopathic medicine. Entropy or spontaneous energy is the negative form of energy, causing heat to accumulate, while potential energy is the highest form of positive energy that manifests itself in the form of work. Consequently, entropy represents the negative and passive side of the matter, while potential energy represents the positive and active side. They are in inverse proportion, i.e., when entropy increases, potential energy decreases, and vice versa. Entropy and potential energy are interrelated; the existence of one is inextricably linked to the existence of the other. Despite their constant motion, entropy moves variably in all directions, whereas potential energy moves linearly and in an orderly manner. From the mind-body perspective, knowledge, human values, moral values, spiritual values, and supporting information enrich potential energy, while material values and distracting information enrich entropy. For example, human value is enhanced when voluntarily donations are given to poor people, and as a result potential energy is increased (voluntarily acquired energy). However, forceful or involuntarily monetary contributions, such as those paid as bribes, increase entropy (involuntarily acquired energy).
Although both behaviours are psychological traits, one builds a compatible system and creates a stronger connection between concept and behaviour, creating a positive aura in the body and thus increasing the entanglement of waves, generating synergy, and enriching potential. In contrast, the other is incompatible with human nature and leaves a negative aura and decreases entanglement and increases collisions of particles and entropy. Halalopathy assumes that all functions and information present in the body can be perceived in terms of entropy and potential energy and can therefore be used to improve prevention and recovery [44].
Conclusion
For effective synthetic design, it is crucial to predict the directionality of reactions. According to the second law of thermodynamics, the total entropy of the universe increases in a spontaneous process. Therefore, the entropy changes play an important role in predicting the direction and spontaneity of the reaction. In a chemical reaction, entropy can be predicted from the number of moles and the states of the reactants and products. In biochemical reactions, catabolic reactions are spontaneous, exothermic, and entropy driven. The energy released is stored as potential energy in the form of ATP and dissipated as entropy in the form of heat. In contrast to a catabolic reaction, an anabolic reaction requires energy to take place, is endothermic, and is not spontaneous. Entropy and potential energy are inversely proportional and interdependent. They are in constant motion; for entropy the motion is scattered in all directions, while potential energy moves in an orderly and linear direction. Chemical potential energy is stored in the constitution, configuration, and conformation of the molecule, and displayed in the form of structure, function, and information. In high-entropy molecules, there is less organization in terms of the constitution, configuration, and conformation, which results in greater vibrational, rotational, and translational motions. Entropy and potential energy can be interpreted as a representation of every function and information in the body, allowing a greater understanding of prevention and control.
Acknowledgment
We would like to thank Prof. Loay Awad, Prof. Khaled Abou Hadeed, and Haitham Alzeer for their useful discussion and support.
Ethical Approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest
None.
Article Info
Article Type
Review of the LiteraturePublication history
Received: Thu 29, Sep 2022Accepted: Mon 17, Oct 2022
Published: Mon 31, Oct 2022
Copyright
© 2023 Jawad Alzeer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2022.02.06
Author Info
Corresponding Author
Jawad AlzeerSwiss Scientific Society for Developing Countries, Zurich, Switzerland
Figures & Tables
References
1.
Camacho FF, Lugo
NU, Martínez HC (2015) The concept of entropy, from its origins to teachers. Revista
Mexicana de Física 61: 69-80.
2.
Namdari A, Li Z
(2019) A review of entropy measures for uncertainty quantification of
stochastic processes. Adv Mech Eng 11: 1-14.
3.
Alzeer J (2022)
Halalopathy: Improving Potential Energy and Minimising Entropy offer an
Integrative approach for more Effective Treatment. Medicon Med Sci 2:
21-24.
4.
Wolfgang F, Karl S
(2003) Waste energy usage and entropy economy. Energy 28: 1281-1302.
5.
Xu SZ, Zhao T, Chen
Q, Liang XG, Guo ZY (2022) State functions/quantities in thermodynamics and
heat transfer. Fundam Res 2: 101-107.
6.
Lambert FL (2002)
Entropy Is Simple, Qualitatively. J Chem Educ 79: 1241-1246.
7.
Geller BD, Dreyfus
BW, Gouvea J, Sawtelle V, Turpen C et al. (2014) Entropy and spontaneity in an introductory physics course for
life science students. Am J Phys 82: 394.
8.
Alzeer J (2020)
Entropy and potential energy as a key role of halalopathy in disease prevention
and cure. Longhua Chin Med 3: 20.
9.
Sabirov DS,
Shepelevich IS (2021) Information Entropy in Chemistry: An Overview. Entropy
(Basel) 23: 1240. [Crossref]
10.
Bonchev D (2003)
Shannon’s Information and Complexity. In Complexity: Introduction and
Fundamentals; CRC Press: London, UK, 157-187.
11.
Ahmad M, Helms
V, Lengauer T, Kalinina OV (2015) Enthalpy-Entropy Compensation upon
Molecular Conformational Changes. J Chem Theory Comput 11:
1410-1418. [Crossref]
12.
de Ligny
CL, Alfenaar M, van der Veen NG (1968) The standard chemical free
enthalpy, enthalpy, entropy and heat capacity of hydration of the hydrogen ion,
and the surface potential of water at 25°C. Recueil des Travaux
Chimiques des Pays-Bas 87: 585-598.
13.
Carson E, Watson J (2002)
Undergraduate students’ understandings of entropy and Gibbs free energy. U
Chem Ed 6: 4-12.
14.
Ribeiro M,
Henriques T, Castro L, Souto A, Antunes L et al. (2021) The Entropy
Universe. Entropy (Basel) 23: 222. [Crossref]
15.
Sutcliffe BT,
Woolley RG (2013) The Potential Energy Surface in molecular quantum mechanics. Prog
Theor Chem Phys 27: 3-40.
16.
Lombardi A, Pirani
F, Bartolomei M, Coletti C, Laganà A (2019) Full Dimensional Potential Energy
Function and Calculation of State-Specific Properties of the
CO+N2 Inelastic Processes Within an Open Molecular Science Cloud
Perspective. Front Chem 7: 309. [Crossref]
17.
Yu YB, Privalov PL,
Hodges RS (2001) Contribution of translational and rotational motions to
molecular association in aqueous solution. Biophys J 81: 1632-1642. [Crossref]
18.
Khmelnitsky RA,
Gippius AA (2013) Transformation of diamond to graphite under heat treatment at
low pressure. Phase Transit 87: 175-192.
19.
Hert MDE, Correll
CU, Bobes J, Bakmas MC, Cohen D et al. (2011) Physical illness in patients with
severe mental disorders. I. Prevalence, impact of medications and disparities
in health care. World Psychiatry 10: 52-77. [Crossref]
20.
Annila A,
Baverstock K (2016) Discourse on order vs. disorder. Commun Integr Biol
9: e1187348. [Crossref]
21.
Russell JB, Cook GM
(1995) Energetics of bacterial growth: balance of anabolic and catabolic
reactions. Microbiol Rev 59: 48-62. [Crossref]
22.
Hopp
AK, Grüter P, Hottiger MO (2019) Regulation
of Glucose Metabolism by NAD+ and ADP-Ribosylation. Cells 8: 890. [Crossref]
23.
Alberts B, Johnson
A, Lewis J, Raff M, Roberts K et al. (2002) How Cells Obtain Energy from Food.
Molecular Biology of the Cell. 4th edition. New York: Garland Science.
24.
Bhatla SC, Lal MA
(2018) Respiration. In: Plant Physiology, Development and Metabolism. Springer,
Singapore.
25.
Palazzo P (2021)
Chemical and Mechanical Aspect of Entropy-Exergy Relationship. Entropy
(Basel) 23: 972. [Crossref]
26.
Andrulis ED (2011)
Theory of the origin, evolution, and nature of life. Life (Basel)
2: 1-105. [Crossref]
27.
Alzeer J, Razem FA
(2021) Hypotheses: implementation of Le Chatelier’s principle as a potential
integrative method to prevent and or cure coronavirus. J Public Health
Emerg 5: 1-7.
28.
Kostic MM (2014)
The Elusive Nature of Entropy and Its Physical Meaning. Entropy 16:
953-967.
29.
Alzeer J (2022)
Halalopathy: Revival of Miraculous Cure and Creation of Favourable
Circumstances for Cancer Therapy. Medicon Med Sci 2: 21-28.
30.
Alzeer J (2022)
Halalopathy: Anxiety and Depression from Logic and Energetic
Perspectives. Am J Biomed Sci & Res 16: 378-384.
31.
Li L, Wang L,
Alexov E (2015) On the energy components governing molecular recognition in the
framework of continuum approaches. Front Mol Biosci 2:
5. [Crossref]
32.
Maximova T, Moffatt
R, Ma B, Nussinov R, Shehu A (2016) Principles and Overview of Sampling Methods
for Modeling Macromolecular Structure and Dynamics. PLoS Comput Biol 12:
e1004619. [Crossref]
33.
Ding F, Jha RK,
Dokholyan NV (2005) Scaling behavior and structure of denatured proteins. Structure
13: 1047-1054. [Crossref]
34.
Saintigny Y, Chevalier
F, Bravard A, Dardillac E, Laurent D et al. (2016) A threshold of endogenous
stress is required to engage cellular response to protect against mutagenesis. Sci
Rep 6: 29412. [Crossref]
35.
Baldwin RL, Zimm BH
(2000) Are denatured proteins ever random coils? Proc Natl Acad Sci U S A 97:
12391-12392. [Crossref]
36.
Alzeer J (2022)
Halalopathy: Role of Entropy in the Aging Process. Am J Biomed Sci
& Res 16: 147-154.
37.
Syamala
PPN, Würthner F (2020) Modulation of the Self-Assembly of
π-Amphiphiles in Water from Enthalpy- to Entropy-Driven by Enwrapping
Substituents. Chemistry 26: 8426-8434. [Crossref]
38.
Vantomme
G, Meijer EW (2019) The construction of supramolecular systems. Science 363:
1396-1397. [Crossref]
39.
Beshnova DA,
Pereira J, Lamzin VS (2017) Estimation of the protein–ligand interaction energy
for model building and validation. Acta Crystallogr D Struct Biol 73:
195-202. [Crossref]
40.
Alzeer J (2019)
Halalopathy: A science of trust in medicine. J Integr Med 17:
150-154. [Crossref]
41.
Alzeer J (2018)
Halalopathic: A New Concept in Medicine. J Mol Genet Med 12:
353.
42.
Alzeer J, Hadeed KA
(2020) Halal Certification of Food, Nutraceuticals, and Pharmaceuticals in the
Arab World. In: Laher I. (eds) Handbook of Healthcare in the Arab World. Cham:
Springer.
43. Alzeer J (2021) Permissible Medicine and Rationalization of Halal Pharma. Halalpshere 1: 43-52.
44. Alzeer J (2022) Halalopathy: Stimulation of the Immune System Through Enrichment of Potential Energy. Int J Regenr Med 5: 1-5.
