Journals
Detection of 2-Dodecylcyclobutanone in Low-Dose Irradiated (0.5 Gy) Goose Fat by Triple-Quad GC-MS/MS
A B S T R A C T
Aim of this work was the development of a sensitive procedure to detect low-dose irradiation in the scale of 0.5 Gy to 20 Gy in goose fat by detection of 2-dodecylcyclobutanone (2-DCB). The general procedure to detect 2-alkylcyclobutanone (2-ACB) according to EN 1785 was optimized by using acetonitrile for extraction of 2-DCB from the lipid fraction before clean-up with silica gel. Gas chromatographic separation was realized by a 15 m TG-5 HT column, and detection was done by using a triple-quad tandem-mass spectrometer in SRM-mode. At the irradiation level of 2 Gy, the optimized procedure showed high repeatability (3.9%), a good intermediate precision (20.9%) and an acceptable recovery rate of 57.8%, with 0.25 ng 2-DCB per gram of fat. With this procedure, a reliable proof of 2-DCB was possible for the irradiation intensities 20, 5 and 2 Gy for three mass transitions; for 0.5 Gy, a proof was possible for one mass transition.
Keywords
Limit of detection, 2-ACB, 2-DCB, x-ray irradiation, EN 1785, GC-MS/MS
Introduction
I Use of Ionizing Irradiation
Ionizing irradiation for the purpose of disinfecting food is most commonly applied to susceptible foodstuffs like eggs, poultry or meat, to kill bacteria [1]. For this application, high intensities of irradiation like 5 to 10 kGy are needed, because bacteria are not very sensitive to irradiation. Lower intensities of irradiation are required (around 0.1 kGy) to control pest populations like insects in fruits, or to inhibit sprouting in e.g., potatoes or onions. Higher doses may cause product alteration, enzyme activity may be influenced by ionizing irradiation [1, 2]. Low doses (below 0.5 Gy) are used in measuring and inspection devices, e.g., to identify foreign bodies in the final check of readily packed products [3].
II Labeling and Verification
Irradiated foodstuff has to be labeled according to legislation. To comply with formalities, methods are needed to verify the use or absence of irradiation [4]. Several methods were developed since 1980, like the electron spin resonance (ESR) spectroscopy to detect radiation-induced radicals, thermo-luminescence or photoluminescence [5]. Especially for fat-containing products, the detection of radiation-induced alkanes and 2-alkylcyclobutanone has become a commonly used procedure [1, 6, 7]. Because 2-ACB was up to date only found in radiated products, it seems to be the most specific and best-suited procedure to proof irradiation [8]. The concentration of 2-ACB is known to be linear to irradiation intensity; per millimole of fatty acids (bound in triglycerides), 1-1.6 nanomole 2-ACB is built [9, 10]. For non-irradiated foods containing a low quantity of irradiated ingredients, it may be of importance to detect also low concentrations of 2-ACB [11].
III Procedures to Determine 2-ACB
i General Procedure
Determination of 2-ACB follows several steps of processing:
i. Sample preparation, including crushing and homogenization.
ii. Extraction of the lipid fraction containing the 2-ACB from the residual sample.
iii. Clean-up and concentration of the lipid fraction.
iv. Separation by gas- (GC) or liquid-chromatographic (LC) procedures.
v. Detection by mass-spectrometric devices (MS or MS/MS) or flame ionization detector (FID).
According to the official procedure, Soxhlet-extraction has to be used, clean-up has to be done with a Florisil column, followed by concentration of the eluate [7, 12]. GC separation was followed by mass-spectrometric detection in the single ion monitoring (SIM) modus at m/z 98 and m/z 112.
ii Improvements of the Procedure
Several improvements of this procedure were carried out, like the reduction of solvents by using supercritical fluid extraction (SCF) or enhancement of time efficiency by accelerated solvent extraction (ASE) or direct solvent extraction (DSE) [13-19]. To improve clean-up, Ndiaye et al. used additionally to the Florisil column, an ion exchange column, impregnated with silver ions, so that disruptive signals in the GC-MS chromatograms were reduced [20]. In this case, the used amount of fat (the equivalent of the sample) may be enlarged. Another way to enlarge sample equivalent by the factor of 10 was used by Horvatovich et al. by using a column with 60 g of silica gel for clean-up instead of the Florisil column [11]. Detection was realized by mass spectroscopy after chemical ionization with butanone so that improved selectivity was reached, and chromatograms showed less disruptive elements. The sensitivity of this procedure was proven by using rice, irradiated with 100 Gy.
Further investigations were done by Sin et al., who tried to reduce disruptions in the ion chromatograms without expensive sample preparations, by derivatization of 2-ACB with pentafluorphenylhydrazine, or by Soncin et al., who tried to avoid a sample preparation, by evaporating the water diluted sample and detecting 2-ACB by gas-chromatography and mass-spectrometry (GC/MS) coupled with solid-phase microextraction (SPME) technique (HS-SPME-GC/MS) [21, 22]. But sensitivity was not improved by this. For Ye et al., high-performance liquid chromatography, coupled with tandem mass spectrometry (HPLC-MS/MS), seemed to be suitable to analyze 2-ACB [23]. They realized that 2-ACB shows as ketone only a very low sensitivity to electrospray ionization; thus a derivatization was done with hydroxylamine. The resulting oxime (2-dodecylcyclobutanone, 2-DCB) showed the desired detection sensitivity, so irradiation could be detected even at very low doses (10 Gy) in chicken meat. Recent investigations used irradiation intensities of 100 Gy in cashew nuts or 400 Gy in nutmeg, lower intensities as used by Ye et al. were not studied yet [23-25].
IV Aim of Investigation
The aim of the investigation was to detect small amounts of 2-DCB in fat-containing food that was treated at low (0.5 Gy) irradiation doses, to show that detection of 2-DCB is also possible on a low concentration level. To reach this low detection limit, the EN 1785 was adapted and varied by optimizing the extraction of 2-DCB from the lipid fraction and optimizing the clean-up process, and highly sensitive triple-quad mass-spectroscopic techniques were used for detection [12].
Materials and Methods
I Chemicals and Reagents
Cyclododecanone was purchased from Acros Organics, Geel, Belgien, Diisobutyl phthalate, Tetradecylcyclobutanone-d29 (2-TCB-D29), Hexahydrofarnesyl acetone and 2-Dodecylcyclobutanone were purchased from Sigma-Aldrich (Fluka), Saint Louis, MO, USA.
II Preparation of Irradiated Samples
As a homogenous and pure fat food matrix, goose fat was chosen and purchased at the local supermarket. Three kg of goose fat was homogenized, and filled in 300 clean PS-petri dishes of 6 cm of diameter, each containing around 10 g of goose dripping, corresponding with a filling level of around 0.5 cm. Irradiation took place at the TU Darmstadt, Germany, by using an X-ray tube (Goliath Titan Isovolt 160E; GE Measurement & Control, Ahrensburg, Germany). Different irradiation intensities were realized by changing amperage and time of irradiation (Table 1). Per irradiation intensity, 50 petri dishes were irradiated. Irradiation intensities were 20, 5, 2, 0.5 and 0 Gy (control). For time-efficient use of the tube, always 2 dishes (at level 0.5 Gy) or 4 dishes (at level 2, 5 and 20 Gy) were treated together. Control dishes were not treated but handled in the same way as treated ones. After treatment, samples were pooled per irradiation intensity in glass vessels to obtain homogenous and sufficient material for subsequent analyses. The irradiated samples were stored at -18°C.
Table 1: Details of irradiation settings for X-ray tube Goliath Titan Isovolt 160E. Mean distance to irradiation source was 39 cm. Irradiation time was calculated on the basis of the irradiation intensity of the X-ray tube for 90kV at 39 cm distance, which was 2.97 Gy/min at 19mA and 5.198 Gy/min at 33.7 mA.
|
Irradiation dose (Gy) |
Voltage (kV) |
Amperage (mA) |
Time (s) |
Filtration |
|
20.0 |
90 |
33.7 |
434 s |
aluminium sheet, 1mm thick |
|
5.0 |
90 |
33.7 |
109 s |
|
|
2.0 |
90 |
33.7 |
43 s |
|
|
0.5 |
90 |
19.0 |
19 s |
III Extraction and Clean-Up Process
The extraction of 2-DCB from the lipid fraction was done according to Hijaz et al. with acetonitrile [19]. The extraction was repeated consecutively for six times, using 20 ml acetonitrile per extraction step. Column clean-up was done as described by Horvatovich et al., but only 2.4 g silica gel was used [11]. To reduce analyte losses during evaporation, 1 µg cyclododecanone was added as keeper during the extraction of the 2-DCB from the lipid fraction, and 0.5 µg diisobutyl phthalate (DIBP) was added as keeper after clean-up with silica gel, before further evaporation.
Using this clean-up process, from 10 g fat, a total of 0.5 ml suitably cleaned extract was obtained. The keeper concentration in the resulting 0.5 ml solution was 2000 ng/ml cyclododecanone and 1000 ng/ml DIBP; thus a surplus of 400-fold and 200-fold were obtained in relation to the 2-DCB content (5 ng/ml solvent) in the sample irradiated with 2 Gy. Sample preparation, including extraction of 2-DCB from the lipid fraction and clean-up, was carried out four times per sample for validation measurements.
IV GC-MS/MS-Analysis
A volume of 1.5 µL was vaporized, the initial temperature of the injector (PTV; Thermo Fisher Scientific, Waltham, MA, USA) of 75 °C increased to 280 °C with a rate of 10 °C sec-1 A 15 m GC column (TG-5HT, 0.25 mm i.d. x 0.25 µm film, Thermo Fisher Scientific, Waltham, MA, USA) was used for gas chromatographic separation, installed in a Trace GC-Ultra (Thermo Fisher Scientific, Waltham, MA, USA). Oven temperatures were 100 °C (for 1.1 minutes), 135 °C, 183 °C, 215 °C and 290 °C (for 5 minutes). Argon was used as collision gas at a pressure of 1.2 mTorr.
Detection was realized by using a triple-quad tandem-mass spectrometer of high sensitivity with two hyperbolic quadrupole rods, each of 25 cm length (TSQ Quantum XLS Ultra, Thermo Fisher Scientific, Waltham, MA, USA), in the selected reaction monitoring (SRM) mode with electron impact ionization (EI) at standard energy (70 eV). A timed-SRM modus with a low cycle time of 0.3 s for a high chromatographic resolution and dwell times of 30 ms per transition were applied. To follow possible retention time variation, specific SRM-tracks of the matrix-own marker hexahydrofarnesyl acetone were used, according to Horvatovich et al., as well as those of DIBP [14]. For quantification, deuterated 2-tetradecylcyclobutanone (2-TCB-D29) was used as an internal standard, which was added at the beginning of the sample preparation.
V Method Development and Performance
i Extraction Process
To proof the extraction efficiency of acetonitrile for 2-DCB from the lipid fraction, several consecutive extracts were prepared from one goose fat sample, irradiated with 20 Gy. 10 g of this sample was added by 20 ml of acetonitrile, and mixing was done for 10 min in a shaker (MultiReax, Heidolph Instruments GmbH&Co. KG, Schwabach, Germany). The solvent was removed, and extraction was repeated eight times; thus, nine separate extracts from one sample were obtained. The first three extracts were pooled, and also the 4th to 6th and the 7th to 9th; thus three pooled extracts were obtained to check, how often times extraction has to be repeated to reach complete extraction.
ii Specificity of Detection
The specificity of the procedure was ensured by using the SRM-mode. Three transitions (m/z 112>98, 98>97 and 98>83) were used to ensure specificity. Matrix related variation of retention time was monitored by using markers like the matrix inherent hexahydro farnesyl acetone and the keeper DIBP.
iii Performance of the Method
The recovery rate was determined by adding 2.5 ng 2-DCB to 10 g control sample (non-treated goose fat) either before or after the extraction and clean-up process; thus losses during sample preparation could be estimated. Repeatability was calculated as a coefficient of variation (relative standard deviation, RSD) of four separately prepared samples per irradiation intensity. Intermediate precision was determined for the irradiation intensities 2 Gy and 5 Gy by comparing the results as the day-to-day variation of 4 replicates, at two different days independently prepared and measured.
Results and Discussion
I Optimizing the Extraction and Clean-up
To improve the extraction of 2-DCB from the lipid fraction, direct solvent extraction with acetonitrile was used, and the extraction was repeated nine times. In the pooled extracts No. 1-3 and No. 4-6, 2-DCB was found, in extract No. 7-9, no 2-DCB was detectable. We concluded that the first six consecutive extractions per sample were sufficient, containing the extractable amount of 2-DCB of the sample. In consequence, further extract preparations were done for 6 consecutive times per sample, and measuring was done with pooled extracts. These extracts were cleaned up by using silica gel, according to Horvatovich et al. [11]. To avoid losses during the evaporation steps keepers (cyclododecanone and DIBP) were used. These optimizations resulted in a concentration of 20:1, which means 10 g fat resulted in 0.5 ml solution.
II Optimizing the Detection of 2-DCB
To improve the selectivity of the detection of 2-DCB SRM transitions were used instead of monitoring single ions (SIM). Because of the high fragmentation rate of the 2-DCB at the applied electron impact ionization, masses of 112 and 98 were used as precursors. The transitions 112>98, 98>97 and 98>83 were chosen because they showed the best selectivity and signal to noise ratio. Even with these transitions, the chromatograms showed several peaks. Therefore, it was necessary to determine which peak showed the 2-DCB. Retention time markers were used to check retention time variations of the 2-DCB due to the influence of the matrix. With the use of the matrix inherent hexahydro farnesyl acetone and the keeper DIBP as markers, a sure location of the 2-DCB-signal was possible even in low dose samples.
III Performance of the Analytical Method
i Linearity and Calibration
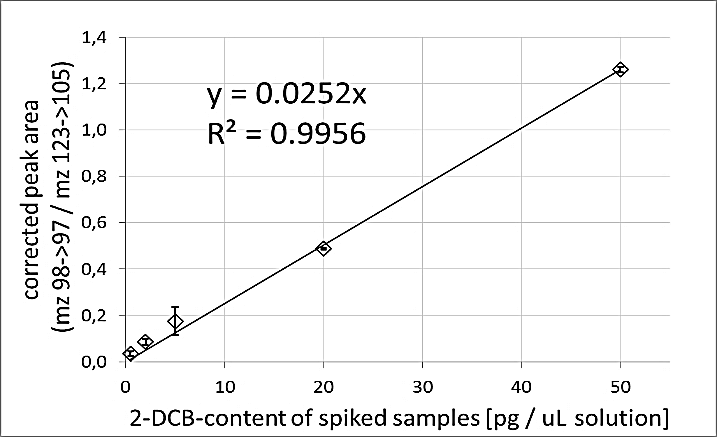
For testing the linearity of the method, two steps were performed. In the first step, spiked samples were obtained by adding 2-DCB in different concentrations to cleaned extracts of non-irradiated samples. These solutions were measured, and the peak areas of the signal were corrected to the internal standard. The corrected peak areas of the signal showed a linear relation to the concentration of 2-DCB with R2 = 0.9989 over the concentration range of 0.5 – 50 ng/mL (Figure 1) and were used as calibration for the determination of the 2-DCB content in the sample.
In the second step, irradiated samples with different radiation intensities were extracted. After cleanup, the extracts were measured, and the content of 2-DCB was determined from the calibration. The determined concentration of 2-DCB in relation to the irradiation intensity is shown in (Figure 2). A linear relation between applied radiation and 2-DCB content was found, with R2 = 0.9993. The amount of 2-DCB, which was formed in the fat due to the irradiation, can be derived from the slope in (Figure 2). Our result of 114.1 ng 2-DCB per gram fat per kGy of X-ray irradiation is in agreement with the results of Ye et al. and Tewfik et al., which reported 104.7 and 110 ng 2-DCB per gram fat per kGy, respectively [23, 26].
Figure 1: Linear correlation between 2-DCB-content of spiked samples and their corrected peak area. Whiskers show standard deviation of three replicates.
Figure 2: Relation of irradiation intensity to measured 2-DCB content in goose fat. Whiskers show standard deviation of four replicates.
ii Precision and Trueness
Repeatability of results was tested per irradiation intensity, each with four separately prepared extracts (replicates). Coefficient of variation per irradiation intensity was 2.8%, 3.9%, 9.7% and 19.9% for 20 Gy, 5 Gy, 2 Gy and 0.5 Gy, respectively, and intermediate precision for irradiation intensities 5 Gy and 2 Gy were 18.5% and 20.9%, respectively, determined as the day-to-day variation of four replicates each. The recovery rate of the improved extraction procedure was 57.8% at a level of 5 ng 2-DCB per ml of the readily prepared solution, which corresponds to the level of the 2-DCB content in the sample irradiated with 2 Gy.
iii Sensitivity
In all irradiated samples, for mass transition 98>97, a clear signal could be found at the retention time of the 2-DCB (Figure 3). For samples with >0.5 ng/mL 2-DCB in the goose fat extracts after clean-up, the other qualifying transitions (112>97 and 98>83) were found, too; thus, a reliable proof of 2-DCB in samples irradiated with 2 Gy or more was possible for three mass transitions, containing approximately 0.25 ng of 2-DCB per g fat or more. In low-dose irradiated samples (0.5 Gy), 2-DCB was detectable only for one transition (98>97), disturbances of the chromatogram occurred for the other transitions; thus, a determination of concentrations lower than 0.25 ng/g fat was possible but lacked specificity in detection.
Figure 3: Part of the SRM-mass track 98>97, used for quantification of 2-DCB content in goose fat. Differences between irradiation intensities are obvious (by different peak sizes).
Conclusion
Optimization of the extraction procedure was possible by using acetonitrile and keeper, and optimization of the detection was possible by highly sensitive triple-quad tandem-mass spectrometry and the use of markers for retention time variation. The optimized procedure showed a recovery rate for 2-DCB of 57.8%, and a repeatability of 9.7% RSD (20.9% intermediate precision) at a level of 5 ng/ml, which was the level found in samples irradiated with 2 Gy, containing approximately 0.25 ng of 2-DCB per g fat. Even in low-dose irradiated samples at the level of 0.5 Gy, 2-DCB was detectable in goose fat with transition 98>97, disturbances of the chromatogram occurred for the other transitions.
A linear relation could be shown between the irradiation intensity and content of 2-DCB. The level of formed 2-DCB in relation to the irradiation intensity was the same as reported by Ye et al. and Tewfik et al. [23, 26]. It was shown that this relation could also be found in samples irradiated at very low doses like 2 Gy and below.
Funding
This work was supported by the Software AG - Stiftung, Darmstadt, Germany [grant number P10355].
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 11, May 2020Accepted: Fri 29, May 2020
Published: Wed 10, Jun 2020
Copyright
© 2023 Peter Stolz. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2020.02.01
Author Info
Gesine Mandt Jenifer Wohlers Peter Stolz Roya Bornhütter Uwe Geier
Corresponding Author
Peter StolzForschungsinstitut KWALIS gGmbH, Dipperz, Germany
Figures & Tables
Table 1: Details of irradiation settings for X-ray tube Goliath Titan Isovolt 160E. Mean distance to irradiation source was 39 cm. Irradiation time was calculated on the basis of the irradiation intensity of the X-ray tube for 90kV at 39 cm distance, which was 2.97 Gy/min at 19mA and 5.198 Gy/min at 33.7 mA.
|
Irradiation dose (Gy) |
Voltage (kV) |
Amperage (mA) |
Time (s) |
Filtration |
|
20.0 |
90 |
33.7 |
434 s |
aluminium sheet, 1mm thick |
|
5.0 |
90 |
33.7 |
109 s |
|
|
2.0 |
90 |
33.7 |
43 s |
|
|
0.5 |
90 |
19.0 |
19 s |


References
- Chauhan SK, Kumar R, Nadanasabapathy S, Bawa AS (2009) Detection Methods for Irradiated Foods. Compr Rev Food Sci Food Saf 2009: 4-16.
- Kader AA (1986) Potential applications of ionizing radiation in postharvest handling of fresh fruits and vegetables. Food Techn 40:117-121.
- EU 1999/2: Directive 1999/2/EC of the European Parliament and of the Council of 22 February 1999 on the approximation of the laws of the Member States concerning foods and food ingredients treated with ionising radiation. Official J L 66: 16-23.
- Kwon JH, Nam KC, Lee EJ, Ahn DU (2011) Evaluation of Radiation-Induced Compounds in Irradiated Raw or Cooked Chicken Meat During Storage. Poult Sci 90: 2578-2583. [Crossref]
- Marchioni E, Horvatovich P, Ndiaye B, Miesch M, Hasselmann C (2002) Detection of low amount of irradiated ingredients in non-irradiated precooked meals. Radiat Phys Chem 63: 447-450.
- Anonymous (2004) Untersuchung von Lebensmitteln – Nachweis von bestrahlten fetthaltigen Lebensmitteln. Gaschromatographische Untersuchung auf Kohlenwasserstoffe - gemäß DIN EN 1784, 2003. Analysis of foodstuffs – Proof of fat containing irradiated foods. Gas chromatographic analysis of alkanes. Amtliche Sammlung von Untersuchungsverfahren nach §35 LMBG. Technisches Komitee TC 275 des CEN (European Committee for Standardization).
- Breidbach A, Ulberth F (2016) Comparative Evaluation of Methods for the Detection of 2-alkylcyclobutanones as Indicators for Irradiation Treatment of Cashew Nuts and Nutmeg. Food Chem 201: 52-58. [Crossref]
- Horvatovich P, Miesch M, Hasselmann C, Delincée H, Marchioni E (2005) Determination of Monounsaturated Alkyl Side Chain 2-alkylcyclobutanones in Irradiated Foods. J Agric Food Chem 53: 5836-5841. [Crossref]
- Gadgil P, Smith JS, Hachmeister KA, Kropf DH (2005) Evaluation of 2-dodecylcyclobutanone as an Irradiation Dose Indicator in Fresh Irradiated Ground Beef. J Agric Food Chem 53: 1890-1893. [Crossref]
- Ndiaye B, Jamet G, Miesch M, Hasselmann C, Marchioni E (1999) 2-Alkylcyclobutanones as markers for irradiated foodstuffs II. The CEN (European Committee for Standardization) method: field of application and limit of utilization. Radiat Phys Chem 55: 437-445.
- Horvatovich P, Werber D, Jung S, Miesch M, Delincee H et al. (2006) Determination of 2-alkylcyclobutanones With Electronic Impact and Chemical Ionization Gas Chromatography/Mass Spectrometry (GC/MS) in Irradiated Foods. J Agric Food Chem 54: 1990-1996. [Crossref]
- Anonymous (2001) Foodstuffs. Detection of irradiated food containing fat. Gas chromatographic/mass spectrometric analysis of 2-alkylcyclobutanones. EN 1785. European Committee for Standardization. Brussels (B). Revised 2003.
- Stewart EM, McRoberts WC, Hamilton JT, Graham WD (2001) Isolation of Lipid and 2-alkylcyclobutanones From Irradiated Foods by Supercritical Fluid Extraction. J AOAC Int 84: 976-986. [Crossref]
- Horvatovich P, Miesch M, Hasselmann C, Marchioni E (2002) Supercritical Fluid Extraction for the Detection of 2-dodecylcyclobutanone in Low Dose Irradiated Plant Foods. J Chromatogr A 968: 251-255. [Crossref]
- Obana H, Furuta M, Tanaka Y (2006) Detection of 2-alkylcyclobutanones in irradiated meat, poultry and egg after cooking. J Health Sci 52: 375-382.
- Obana H, Furuta M, Tanaka Y (2007) Effects of temperature during irradiation on the production of 2-alkylcyclobutanones in beef. J Health Sci 53: 215-219.
- Kumar A (2008) Food irradiation and development of an alternative method for the detection of 2-alkylcyclobutanone. Master Thesis, Kansas State University, Manhattan, Kansas.
- Hijaz F (2010) Metabolism and formation of 2-dodecylbutanone in irradiated ground beef. Dissertation, Food Science, Kansas State University, Manhattan, Kansas.
- Hijaz F, Kumar A, Smith JS (2010) A Rapid Direct Solvent Extraction Method for the Extraction of 2-dodecylcyclobutanone From Irradiated Ground Beef Patties Using Acetonitrile. J Food Sci 75: T118-T122. [Crossref]
- Ndiaye B, Horvatovich P, Miesch M, Hasselmann C, Marchioni E (1999) 2-Alkylcyclobutanones as Markers for Irradiated Foodstuffs. III. Improvement of the Field of Application on the EN 1785 Method by Using Silver Ion Chromatography. J Chromatogr A 858: 109-115. [Crossref]
- Sin DW, Wong Y, Yao MW (2006) Application of pentafluorophenyl hydrazine on detecting 2-dodecylcyclobutanone and 2-tetradecylcyclobutanone in irradiated chicken, pork and mangoes by gas chromatography-mass spectrometry. Eur Food Res Technol 222: 674-680.
- Soncin S, Panseri S, Rusconi M, Mariani M, Chiesa LM et al. (2012) Improved determination of 2-dodecylcyclobutanone in irradiated ground beef patties by gas-chromatography–mass-spectrometry (GC/MS) coupled with solid-phase microextraction (SPME) technique. Food Chem 134: 440-444.
- Ye Y, Liu H, Horvatovich P, Chan W (2013) Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometric Analysis of 2-alkylcyclobutanones in Irradiated Chicken by Precolumn Derivatization With Hydroxylamine. J Agric Food Chem 61: 5758-5763. [Crossref]
- Breidbach A, Ulbert F (2016) Comparative Evaluation of Methods for the Detection of 2-alkylcyclobutanones as Indicators for Irradiation Treatment of Cashew Nuts and Nutmeg. Food Chem 201: 52-58. [Crossref]
- Zanardi E, Caligiani A, Novelli E (2018) New insights to detect irradiated food: An Overview. Food Anal Methods 11: 224-235.
- Tewfik IH, Ismail HM, Sumar S (1998) A rapid supercritical fluid extraction method for the detection of 2-alkylcyclobutanones in gamma-irradiated beef and chicken. LWT Food Sci Technol 31: 366-370.
