Desmoplastic Small Round Cell Tumor with an Unusual Site and Age Presentation: A Case Report and Literature Review
Desmoplastic Small Round Cell Tumor with an Unusual Site and Age Presentation: A Case Report and Literature Review
A B S T R A C T
Desmoplastic small round cell tumor (DSRCT) is a rare, aggressive sarcoma usually arising in the abdomen or pelvis of young males under 30 years of age. We present a case of a 32-year-old male with a palpable axillary mass and multiple bilateral lung nodules. Excisional biopsy of the axillary mass showed sheets and nests of small round cells with numerous mitoses, areas of necrosis, and desmoplastic stroma. Initial immunohistochemical stains performed at an outside institution demonstrated immunoreactivity for epithelial markers and focal reactivity for breast markers. The tumor was initially diagnosed as poorly differentiated carcinoma, with consideration of a possible primary breast cancer. Additional workup demonstrated strong, diffuse positivity for desmin in tumor cells, leading to the final diagnosis of DSRCT. Subsequent molecular testing confirmed DSRCT with EWSR1 gene rearrangement. This case illustrates the importance of recognizing the morphologic features of DSRCT in the setting of uncommon location or patient age.
Keywords
Carcinoma, desmin, desmoplasia, EWSR1 gene rearrangement, small round cell tumor
Introduction
Desmoplastic small round cell tumor (DSRCT) is a rare, aggressive malignant mesenchymal tumor first described as a distinct entity by Gerald and Rosai in 1989 [1]. Epidemiology and histogenesis of the tumor have not been well established because of the limited number of reported cases. The mean reported patient age is 22 years, with a wide range of 6 to 49 years of age. However, an occurrence at age over 30 years is extremely rare. The male to female ratio is 4:1 [1]. The most common locations are the abdomen and pelvis with early metastasis to the lung or liver. DSRCT has rarely been reported at unusual sites such as the head and neck, intracranial, thigh, axilla/shoulder, inguinal/paratesticular, intraosseous areas, and uterine corpus [2-4].
Histologically, DSRCT presents as clusters of small blue cells in a background of the abundant desmoplastic stroma. The tumor cells are typically immunoreactive for keratin, epithelial membrane antigen, vimentin, desmin, neuron-specific enolase, and EWS-WT1 chimeric protein. DSRCT is generally negative for actin, myogenin, and chromogranin by immunohistochemistry [3]. This immunohistochemical staining pattern indicates epithelial, mesenchymal, and neural phenotypic components of the tumor. The classic chromosomal translocation t(11;22) (p13; q12) found in DSRCT forms a fusion protein from the Ewing’s sarcoma gene EWS to the Wilms’ tumor suppressor gene WT1 [1]. Because of its rare occurrence and a lack of clear consensus on diagnostic criteria, DSRCT is often misdiagnosed as poorly differentiated carcinoma at initial evaluation, especially when it presents at unusual locations or in patients over 30 years of age. We present a challenging case of DSRCT with unusual clinical presentation and its associated immunoprofile.
Case Presentation
A 32-year-old male with no significant past medical history presented with a 4.5 cm non-tender, palpable left axillary mass that had grown over the past five months. The patient denied weight loss, fever, night sweats, or shortness of breath. Chest computed tomography (CT) revealed multiple bilateral lung nodules (up to 2 cm) and a massive left axillary adenopathy with bilateral hilar and mediastinal adenopathy. CT scan also exhibited a sub-centimeter round nodule in the right breast, consistent with gynecomastia. Abdomen and pelvis CT scans were unremarkable.
Excisional biopsy of the left axillary mass was performed and revealed a high-grade undifferentiated neoplasm with small round tumor cells and intense desmoplastic stromal reaction. Initial workup at an outside institution showed the tumor expressed epithelial markers (MOC-31, AE1/3, and E-cadherin) with focal and weak reactivity for breast markers [GATA3 and estrogen receptor (ER)]. Based on these findings, the initial diagnosis rendered was metastatic poorly differentiated carcinoma, with a consideration of a primary breast cancer. The patient came to our institution for treatment. Review of the external pathology and fluorescent in situ hybridization (FISH) for HER2 was requested by the treating oncologist at our institution.
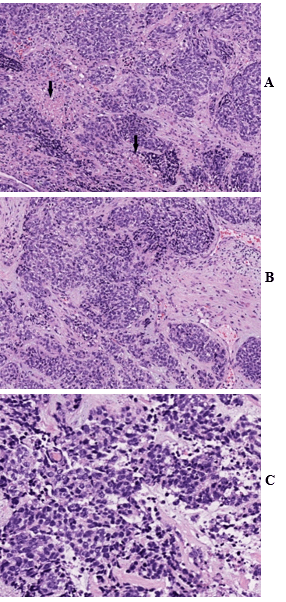
Figure 1: A) Hematoxylin and eosin (H&E) stain show sheets and nests of small round tumor cells with prominent desmoplastic stroma and areas of necrosis (arrow). B) & C) High magnification demonstrates small round tumor cells with scanty cytoplasm and hyperchromatic nuclei. H&E stain, A) 100X; B) 200X; C) 400X.
The left axillary mass biopsy submitted for review showed sheets and nests of small round tumor cells associated with an intense desmoplastic stromal reaction. The tumor cells had scant cytoplasm, hyperchromatic nuclei, numerous mitoses, and scattered areas of tumor necrosis (Figures 1A, 1B & 1C). Very focal lymphoid tissue was identified at the periphery, representing a lymph node effaced by the metastatic tumor.
Review of the external immunohistochemical stains showed diffuse positive immunostaining for MOC-31, AE1/3, and E-cadherin. There was focal and weak positive immunostaining for CK20, GATA-3, and ER. The tumor cells were negative for CK7, CK5, CK 34βE12, p63, CDX2, TTF1, Napsin-A, S100 protein, LCA (CD45), CD3, CD20, synaptophysin, chromogranin, PAX-8, NKX3.1, HER2/neu, and progesterone receptor (PR) (Table 1). Flow cytometry was negative for lymphoproliferative disorders.
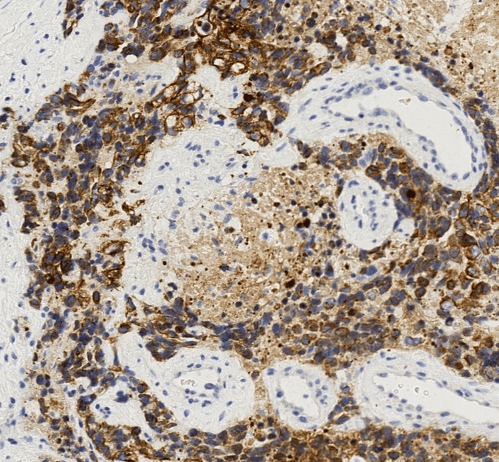
Figure 2: The tumor cells display strong and diffuse reactivity to cytokeratin. CK AE1/3 immunohistochemical stain, 400X.
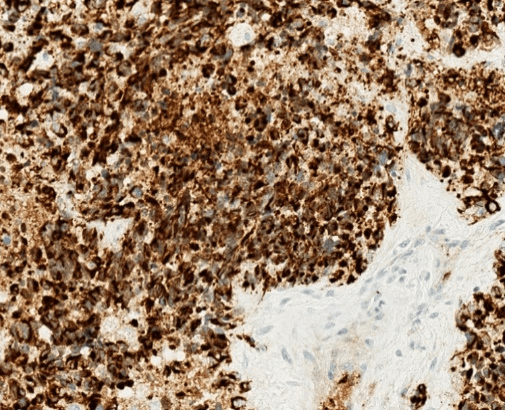
Figure 3: Immunohistochemical stain for desmin shows strong and diffuse staining with a perinuclear dot-like pattern. Desmin immunohistochemical stain, 400X.
Although metastatic breast carcinoma was suggested by the findings of an axillary mass, contralateral breast mass and weak immunohistochemical staining for breast markers, the patient’s young age, male gender, absent ipsilateral breast mass, and extensive desmoplastic stromal reaction indicated a strong possibility for an alternative diagnosis. Despite the unusual location and age, DSRCT was suspected based on the presence of small round tumor cells, prominent desmoplastic stroma, and epithelial cell immunoreactivity. Subsequent immunohistochemical stains for cytokeratin using the AE1/3 antibody and for desmin showed strong diffuse positivity in tumor cells for both markers (Figures 2 & 3). The overall histological and immunohistochemical findings were diagnostic of DSRCT. Molecular testing was subsequently performed and showed an EWSR1 gene rearrangement, further supporting the diagnosis of DSRCT. HER2 FISH test was not performed. We interpreted this tumor as most likely arising in the axillary soft tissue with direct extension into the adjacent axillary lymph node.
Table 1: Antibodies used in this study.
|
ABTIBODY |
CLONE |
DILUTION |
CONTROL |
VENDER |
PLATFORM |
|
MOC-31 |
MOC-31 |
RTU |
Colon |
Ventana |
Ventana Ultra |
|
AE1/3 |
AE1/AE3/pk26 |
RTU |
Skin |
Ventana |
Ventana Ultra |
|
E-cadherin |
36 |
RTU |
Breast |
Ventana |
Ventana Ultra |
|
CK7 |
RN7 |
1:100 |
Breast |
Leica |
Leica Bond |
|
CK20 |
Ks20.8 |
1:50 |
Colon |
Leica |
Leica Bond |
|
CK5 |
D5/16B4 |
RTU |
Skin |
Leica |
Leica Bond |
|
HMWCK |
CK34βE12 |
RTU |
Prostate |
Leica |
Leica Bond |
|
GATA-3 |
L50-823 |
RTU |
Breast |
Leica |
Leica Bond |
|
ER |
SP1 |
RTU |
Breast |
Ventana |
Ventana Ultra |
|
PR |
IE2 |
RTU |
Breast |
Ventana |
Ventana Ultra |
|
p63 |
4A4 |
RTU |
Prostate |
Ventana |
Ventana Ultra |
|
CDX2 |
EPR2764Y |
RTU |
Colon |
Leica |
Leica Bond |
|
TTF1 |
8G7G3/1 |
1:200 |
Lung |
Leica |
Leica Bond |
|
Napsin-A |
IP64 |
1:400 |
Lung |
Leica |
Leica Bond |
|
S-100 |
4C4.9 |
RTU |
Melanoma |
Leica |
Leica Bond |
|
LCA (CD45) |
RP2/18 |
RTU |
Tonsil |
Ventana |
Ventana Ultra |
|
CD3 |
LN10 |
1:500 |
Tonsil |
Leica |
Leica Bond |
|
CD20 |
L26 |
1:200 |
Tonsil |
Leica |
Leica Bond |
|
Synaptophysin |
299 |
1:100 |
Pancreas |
Leica |
Leica Bond |
|
Chromogranin |
430 |
1:50 |
Pancreas |
Leica |
Leica Bond |
|
PAX-8 |
Polyclonal |
1:100 |
Thyroid Ca |
Proteintech |
Leica Bond |
|
NKX3.1 |
Polyclonal |
RTU |
Prostate Ca |
Leica |
Leica Bond |
|
HER2/neu |
4B5 |
RTU |
Breast Ca |
Ventana |
Ventana Ultra |
HMWCK: high molecular cytokeratin; ER and PR: estrogen and progesterone receptor; RTU: ready-to-use.
Table 2: Case summary with immunohistochemical and molecular study findings.
|
Clinical presentation |
32-year-old man with 4.5 cm palpable left axillary mass |
|
CT scan |
Multiple bilateral lung nodules Massive left axillary adenopathy Bilateral hilar and mediastinal adenopathy Subcentimeter nodule in the right breast |
|
Immunostains |
Positive for MOC-31, AE1/3, and E-cadherin Weak positive for CK20, GATA-3, and ER Negative for CK7, CK5, CK 34βE12, p63, CDX2, TTF1, Napsin-A, S-100 protein, LCA (CD45), CD3, CD20, synaptophysin, chromogranin, PAX-8, NKX3.1, HER2/neu, and PR |
|
Flow cytometry |
Negative for lymphoproliferative disorders |
|
Molecular testing |
Positive for EWSR1 gene rearrangement |
ER & PR: estrogen and progesterone receptor; LCA: leukocyte common antigen.
After the diagnosis of DSRCT was made, the patient received six cycles of chemotherapy with vincristine/adriamycin/ifosfamide, followed by two cycles of ifosfamide/etoposide. The patient is in stable condition with no significant changes in lymphadenopathy and lung nodules. He is currently undergoing radiation therapy to the axilla and is alive with disease 15 months after the first detection of the axillary mass. The case summary with immunohistochemical and molecular study findings is in (Table 2).
Discussion
This case illustrates that DSRCT can be confused with carcinoma due to its histologic resemblance to the growth patterns of epithelial tumors with nesting arrangement of oval to round tumor cells and desmoplastic stroma. Its diagnosis can be even more challenging when patients present with uncommon clinical features such as atypical sites or ages, as seen in this case.
Our case was initially interpreted as poorly differentiated carcinoma with a consideration of a possible primary breast cancer. Based on the initial pathologic findings, our patient was considered to have probable breast carcinoma presenting with axillary nodal metastasis. Immunoreactivity for epithelial markers and focal immunoreactivity for breast markers added further confusion to this case. Several findings in the case, however, raised a concern about the initial diagnosis. First, male breast cancer is rare and constitutes only about 1% of malignancies in men. It commonly occurs in patients over 60 years of age and is exceedingly rare in young men, especially with axillary metastasis. Second, the patient did not have an ipsilateral breast lesion, and the contralateral breast lesion showed benign features on an imaging study. Third, focal weak immunostaining for GATA-3 and ER may be seen in many tumors or benign lesions and does not constitute diagnostic evidence for breast cancer, as do other epithelial markers. Fourth, a negative stain for CK7 strongly argues against a diagnosis of breast cancer, as CK7-negative breast cancer is extremely rare [5].
DSRCT presenting in patients over 30 years of age in tumor locations outside of the abdominal cavity/pelvis, as seen in this case, is rare. Without recognizing the features of DSRCT or performing adequate ancillary testing, this case would have been misinterpreted as high-grade carcinoma, which could have led to inappropriate treatment. To avoid misdiagnosis of this rare entity, especially in cases with unusual presentations, it is important to keep this entity as a differential diagnosis when a young patient presents with a small round cell tumor with prominent desmoplastic stroma. This is also an excellent example of the importance of interpreting the immunohistochemical stains in the context of morphological findings and clinical presentations. Although positivity for epithelial and breast markers are often supportive of a diagnosis of breast carcinomas, exceptions do exist, as seen in this case. A focal and weak stain for markers critical for a diagnosis, such as GATA-3 in this case, should be interpreted with great caution because these markers may be weakly expressed in other tumors.
On the other hand, missing markers that are classically positive in diagnosis, such as CK7 in this case, should prompt pathologists to search for alternative diagnoses. Histologic diagnosis should never be made based solely on a single or a few immunohistochemical stains without integrating clinical and morphological features. It is not uncommon to reach an incorrect diagnosis by over-interpreting immunohistochemical stains, especially when the reactivity is weak and focal.
Although it is very rare, atypical presentations for DSRCT have been reported. Al-Ibraheemi et al. analysed atypical presentations of 34 cases of DSRCT with uncommon locations and ages and suggested three atypical scenarios with corresponding differential diagnosis [3]. The first scenario describes patients less than 30 years of age with atypical location and a differential diagnosis, including non-Hodgkin’s lymphoma/leukemia, metastatic neuroblastoma, or sarcomas, including Ewing’s sarcoma and rhabdomyosarcoma. The second scenario describes atypical location for patients over 30 years of age with a differential diagnosis of non-Hodgkin’s lymphoma, rhabdomyosarcoma, poorly differentiated carcinoma, or poorly differentiated sarcoma. The third scenario suggests a typical location in patients over 30 years of age with a differential diagnosis of a germ cell tumor, poorly differentiated carcinoma, or mesothelioma. Tao et al. reported a case of a 55-year-old female with sinonasal DSRCT with atypical location and unusual age presentation [6].
Chen et al. reported two cases of DSRCT, including a 25-year-old male who presented with recurrent diarrhea and abdominal distension and was found to have multiple liver masses [7]. Because of the imaging study and laboratory data, this patient was initially diagnosed as having an infection. A biopsy was performed, resulting in an incomplete, vague diagnosis of a poorly differentiated malignant tumor until the proper diagnosis of DSRCT was made. The other case in this report featured a 68-year-old male who presented with consistent abdominal pain and was found to have an abdominal mass. The biopsy of the mass was misdiagnosed as adenocarcinoma initially, but further immunohistochemical stains proved it to be a DSRCT. These examples illustrate the significant challenges when diagnosing DSRCT in patients with uncommon clinical presentations. Because the treatment and prognosis of DSRCT are drastically different from those of other tumor types, careful evaluation of pathologic material with the integration of clinical, morphological, and immunohistochemical findings is critical for achieving an accurate diagnosis. After the diagnosis of DSRCT in this patient was made, he received a sarcoma-based treatment instead of breast cancer therapy.
Various forms of treatment have been used for DSRCT. The P6 protocol (chemotherapy with cyclophosphamide, doxorubicin, vincristine, ifosfamide, and etoposide followed by a stem cell transplant) is an aggressive alkylator-based therapy that prolongs survival [8]. Chemotherapy and complete cytoreductive surgery also improve survival [8]. Hyperthermic intraperitoneal perfusion, in combination with other modalities, may also improve survival; however, further research is required [9]. Whole abdominopelvic radiotherapy has been used in conjunction with chemotherapy and surgery and can prolong survival in metastatic intra-abdominal tumors. However, the hematologic and gastrointestinal toxicities associated with this treatment limit its long-term efficacy [10]. Although, the follow-up period is too short in this case, the patient is still alive with the disease with chemo- and radiotherapy.
Despite improvements in treatment modalities for DSRCT, the overall prognosis for this tumor remains poor. The average five-year survival, even with treatment, is 15% [11]. Neither age, gender of the patient, nor size of the presenting tumor has prognostic significance, though extra-abdominal disease shows improved survival relative to abdominal disease [12].
In conclusion, DSRCT is a rare malignancy that may have unusual clinical presentations. Awareness of possible variations and pursuit of further workup in young male patients with small round cell tumors and prominent desmoplastic stroma is critical to arrive at an accurate diagnosis and guide proper clinical management.
Acknowledgments
The authors would like to thank Dr. Sasha M. Pejerrey and Heather L. McConnell for their editorial assistance with this manuscript.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 20, Jul 2020Accepted: Sat 01, Aug 2020
Published: Thu 27, Aug 2020
Copyright
© 2023 Jae Y Ro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.08.11
Author Info
Moon Joo Kim Renee K Eng Ahmed N Shehabeldin Yimin Ge Mary R Schwartz Yun Wu Jae Y Ro
Corresponding Author
Jae Y RoDepartment of Pathology and Genomic Medicine, Houston Methodist Hospital, Weill Medical College of Cornell University, Houston, Texas, USA
Figures & Tables
Table 1: Antibodies used in this study.
|
ABTIBODY |
CLONE |
DILUTION |
CONTROL |
VENDER |
PLATFORM |
|
MOC-31 |
MOC-31 |
RTU |
Colon |
Ventana |
Ventana Ultra |
|
AE1/3 |
AE1/AE3/pk26 |
RTU |
Skin |
Ventana |
Ventana Ultra |
|
E-cadherin |
36 |
RTU |
Breast |
Ventana |
Ventana Ultra |
|
CK7 |
RN7 |
1:100 |
Breast |
Leica |
Leica Bond |
|
CK20 |
Ks20.8 |
1:50 |
Colon |
Leica |
Leica Bond |
|
CK5 |
D5/16B4 |
RTU |
Skin |
Leica |
Leica Bond |
|
HMWCK |
CK34βE12 |
RTU |
Prostate |
Leica |
Leica Bond |
|
GATA-3 |
L50-823 |
RTU |
Breast |
Leica |
Leica Bond |
|
ER |
SP1 |
RTU |
Breast |
Ventana |
Ventana Ultra |
|
PR |
IE2 |
RTU |
Breast |
Ventana |
Ventana Ultra |
|
p63 |
4A4 |
RTU |
Prostate |
Ventana |
Ventana Ultra |
|
CDX2 |
EPR2764Y |
RTU |
Colon |
Leica |
Leica Bond |
|
TTF1 |
8G7G3/1 |
1:200 |
Lung |
Leica |
Leica Bond |
|
Napsin-A |
IP64 |
1:400 |
Lung |
Leica |
Leica Bond |
|
S-100 |
4C4.9 |
RTU |
Melanoma |
Leica |
Leica Bond |
|
LCA (CD45) |
RP2/18 |
RTU |
Tonsil |
Ventana |
Ventana Ultra |
|
CD3 |
LN10 |
1:500 |
Tonsil |
Leica |
Leica Bond |
|
CD20 |
L26 |
1:200 |
Tonsil |
Leica |
Leica Bond |
|
Synaptophysin |
299 |
1:100 |
Pancreas |
Leica |
Leica Bond |
|
Chromogranin |
430 |
1:50 |
Pancreas |
Leica |
Leica Bond |
|
PAX-8 |
Polyclonal |
1:100 |
Thyroid Ca |
Proteintech |
Leica Bond |
|
NKX3.1 |
Polyclonal |
RTU |
Prostate Ca |
Leica |
Leica Bond |
|
HER2/neu |
4B5 |
RTU |
Breast Ca |
Ventana |
Ventana Ultra |
HMWCK: high molecular cytokeratin; ER and PR: estrogen and progesterone receptor; RTU: ready-to-use.
Table 2: Case summary with immunohistochemical and molecular study findings.
|
Clinical presentation |
32-year-old man with 4.5 cm palpable left axillary mass |
|
CT scan |
Multiple bilateral lung nodules Massive left axillary adenopathy Bilateral hilar and mediastinal adenopathy Subcentimeter nodule in the right breast |
|
Immunostains |
Positive for MOC-31, AE1/3, and E-cadherin Weak positive for CK20, GATA-3, and ER Negative for CK7, CK5, CK 34βE12, p63, CDX2, TTF1, Napsin-A, S-100 protein, LCA (CD45), CD3, CD20, synaptophysin, chromogranin, PAX-8, NKX3.1, HER2/neu, and PR |
|
Flow cytometry |
Negative for lymphoproliferative disorders |
|
Molecular testing |
Positive for EWSR1 gene rearrangement |
ER & PR: estrogen and progesterone receptor; LCA: leukocyte common antigen.
References
- W L Gerald, M Ladanyi, E de Alava, M Cuatrecasas, B H Kushner et al. (1998) Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol 16: 3028-3036. [Crossref]
- Christina K Lettieri, Pamela Garcia Filion, Pooja Hingorani (2014) Incidence and outcomes of desmoplastic small round cell tumor: results from the surveillance, epidemiology, and end results database. J Cancer Epidemiol 2014: 680126. [Crossref]
- Alyaa Al Ibraheemi, Cory Broehm, Munir R Tanas, Andrew E Horvai, Brian P Rubin et al. (2019) Desmoplastic Small Round Cell Tumors With Atypical Presentations: A Report of 34 Cases. Int J Surg Pathol 27: 236-243. [Crossref]
- Han Hsi Wong, Helen M Hatcher, Charlotte Benson, Omar Al Muderis, Gail Horan et al. (2013) Desmoplastic small round cell tumour: characteristics and prognostic factors of 41 patients and review of the literature. Clin Sarcoma Res 3: 14. [Crossref]
- Shaolei Lu, Evgeny Yakirevich, Li Juan Wang, Murray B Resnick, Yihong Wang (2019) Cytokeratin 7-negative and GATA binding protein 3-negative breast cancers: Clinicopathological features and prognostic significance. BMC Cancer 19: 1085. [Crossref]
- Yanli Tao, Lina Shi, Li Ge, Tiejun Yuan, Li Shi (2019) Sinonasal desmoplastic small round cell tumor: a case report and review of the literature. BMC Cancer 19: 868. [Crossref]
- Jiajia Chen, Jifang Sheng, Lijun Wang, Zhao Ming Wang, Lanjuan Li (2015) Desmoplastic small-round-cell tumor of the abdomen: A report of two rare cases. Oncol Lett 10: 705-708. [Crossref]
- Vivek Subbiah, Salah Eddine Lamhamedi Cherradi, Branko Cuglievan, Brian A Menegaz, Pamela Camacho et al. (2018) Multimodality Treatment of Desmoplastic Small Round Cell Tumor: Chemotherapy and Complete Cytoreductive Surgery Improve Patient Survival. Clin Cancer Res 24: 4865-4873. [Crossref]
- Andrea Hayes Jordan, Holly Green, Nancy Fitzgerald, Lianchun Xiao, Peter Anderson (2010) Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. J Pediatr Surg 45: 1000-1006. [Crossref]
- Karyn A Goodman, Suzanne L Wolden, Michael P La Quaglia, Brian H Kushner (2002) Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys 54: 170-176. [Crossref]
- Ajaz Bulbul, Bridget Noel Fahy, Joanne Xiu, Sadaf Rashad, Asrar Mustafa et al. (2017) Desmoplastic Small Round Blue Cell Tumor: A Review of Treatment and Potential Therapeutic Genomic Alterations. Sarcoma 2017: 1278268. [Crossref]
- Imran Hassan, Roman Shyyan, John H Donohue, John H Edmonson, Leonard L Gunderson et al. (2005) Intraabdominal desmoplastic small round cell tumors: a diagnostic and therapeutic challenge. Cancer 104: 1264-1270. [Crossref]
