Current Approaches in Dental Alveolar Abscess in Sight of Bone Related Drugs
A B S T R A C T
Dentoalveolar abscess, a localized accumulation of pus in the alveolar bone, can produce significant changes in alveolar bone proper that may be resulted by dental caries, trauma or pulpitis causing alveolar bone resorption or even loss. Unfortunately, it can extend vertically toward the apex of the tooth. Additionally, a periapical abscess may form in the alveolar bone adjacent to the apex of the tooth. Interestingly, many bacterial products can stimulate osteoclast formation and bone resorption of periapical tissues. This review displayed drugs that can affect bone resorption that classified as (1) Osteoclast inhibitors such as: bisphosphonates, gene therapeutic (Cathepsin K) estrogens, selective estrogen receptor modulators (SERMs), calcitonin monoclonal antibodies such as denosumab and (2) Osteoclast activators such as DMP-PYT, nuclear factor-Kappa B (RANK) signaling and adrenomedullin.
Keywords
Abscess, Osteoclast, osteoblast, bone resorption, drugs
Introduction
Dental or dentoalveolar abscess is a localized accumulation of pus in the alveolar bone apically to the tooth. It may occur secondary to dental caries, trauma, deep fillings or failed root canal treatment. Bacteria and microorganisms entering the periapical tissues via the apical foramen, these bacteria can induce acute inflammation leading to pus formation. The pathogenesis of dentoalveolar abscess is polymicrobial in nature, comprising of various facultative anaerobes, such as the viridans group streptococci and the Streptococcus anginosus group, and strict anaerobes, especially anaerobic cocci, Prevotella and Fusobacterium species [1]. Pulpitis is an inflammation of dental pulp and may also result in alveolar bone loss. It may result from trauma, thermal shock, chemical stimulus or mishaps procedures of dental pulp, but most often reflects invasion of dental caries into the pulp chamber. If inflammatory products come through the pulp canal, it will produce a local inflammatory reaction in the alveolar bone near to the apex of the tooth, called osteomyelitis, which , can result in a periapical abscess [2].
Dental abscess can produce significant changes in alveolar bone proper [2]. Alveolar bone is one of three tissues which support the tooth; the others are the periodontal ligament and the cementum. Alveolar bone is formed by intramembranous bone formation of the mandible and maxilla [1]. Periodontal disease starts as inflammation in the gingival tissues. If it was untreated, it will spread to the PDL and alveolar bone. Therefore, advanced periodontal disease results in significant alveolar bone loss. It may include a single tooth but will include adjacent teeth. Alveolar bone loss can extend vertically toward the apex of the tooth. In this case , a periapical abscess may form in the alveolar bone adjacent to the apex of the tooth. Many bacterial products of some of these microorganisms can stimulate osteoclast formation and bone resorption of periapical tissues [3].
In fact, there was a highly relation between numbers and types of bacteria and number of osteoclastic cells in dental abscess. On the other hand, there was an osteogenic cells which found in the infected dental field to conserve the remaining healthy bone and produce newly bone cells which reduce spread of infection all over the bone of maxilla or mandible [3]. In view of drugs that can affect bone resorption, it could be classified into: Osteoclast inhibitors and Osteoclast activators.
I Osteoclast Inhibitors
Antiresorptive therapies are used to increase bone strength in case of osteoporosis and are classified into: bisphosphonates, gene therapeutic (Cathepsin K) estrogens, selective estrogen receptor modulators (SERMs), calcitonin monoclonal antibodies such as denosumab [4].
i Cathepsin K
A novel approach of gene therapeutic using recombinant adeno-associated virus (AAV)-mediated RNAi knock¬down of Cathepsin K (Ctsk) gene expression, was used to target osteoclasts and periapical bone resorption in vivo. It was reported that AAV-sh- Cathepsin K (AAV-sh-Ctsk) capable to impair osteoclast function in vivo and reduce bacterial infection-stimulated bone resorption by 88% [5]. Interestingly, a decrease in mononuclear leukocyte infiltration and inflammatory cytokine expression was accompanying the reduced periapical lesion size. Literatures revealed that AAV-RNAi silencing of Cathepsin K in periapical tissues can significantly lower bone destruc¬tion, endodontic disease development, and inflammation in the periapical lesion [5].
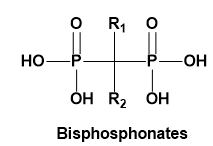
ii Bisphosphonates
Bisphosphonates are a class of drugs that prohibit bone resorption and are involved in treatment of metabolic diseases like osteoporosis, hypercalcaemia of malignancy, Paget’s disease and multiple myeloma [6]. Bisphosphonates chemical structure consists of two phosphate group attached by single carbon atom [7]. This class is classified into: nitrogen containing and non-nitrogen containing type. Although they are the standard treatment of choice for skeletal problems such as osteoporosis, other bone disorders and certain forms of malignancy, the use of antiresorptive therapy has been implicated in the pathoetiology of osteonecrosis of the jaw, which is a painful and debilitating condition [8]. Those containing nitrogen showed potential activity along with accumulation of maximum concentration in the matrix and osteoclasts [9].
The mechanism of action is explained by that bisphosphonates possess a high affinity toward bone minerals and can bind strongly to hydroxyapatite leading to selective uptake to the target organ and high concentration in bone, particularly at the sites of active bone remodeling. Furthermore, they inhibit the osteoclast differentiation, reducing their activity, and inducing osteoclast apoptosis [10]. Currently, there is no effective treatment for bisphosphonate induced osteonecrosis, so prevention is extremely important. Precautions should be taken in consideration with patients who are at the risk of development of osteonecrosis of jaw (ONJ) especially with dental surgical procedure like extractions, retrograde apicoectomies, periodontal surgery and implant placement is contemplated [11].
iii Estrogens
Estrogens can work through blocking cytokine signals in order to activate osteoclasts that in turn suppress bone resorption and leads to increased bone strength. Exogenous estrogens were the main therapy for osteoporosis prevention in postmenopausal women to reduce the risk of vertebral and hip fractures [12]. Literatures reported that women who used hormone replace¬ment therapy had a 33% reduction in vertebral fractures and a 40% reduction in hip fractures compared with control women [13].
iv Selective Estrogen Receptor Modulators (SERM)
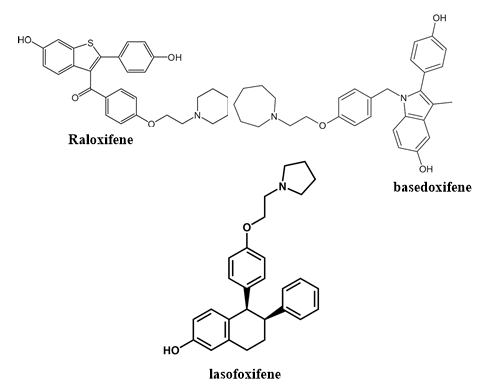
They have estrogen agonist activity at bone to inhibit bone resorption through the same mechanism as do estrogens. Raloxifene is the most widely used SERM in osteo¬porosis treatment [14]. Interestingly, no significant reduction was observed in the risk of fractures at nonvertebral sites in either raloxifene study. The major safety concern of raloxifene therapy is related to its association with increased risks of venous thromboembolism, pulmonary embolism and fatal stroke [15]. Raloxifene treatment was not associated with death from any cause or with the total risk of stroke [14]. Other SERMs, such as basedoxifene and lasofoxifene reduce the risk of vertebral fractures in postmenopausal women with osteoporosis as well as non¬vertebral fractures in women with osteoporosis aged 59–80 years [16, 17].
v Calcitonin
Calcitonin, a 32-amino-acid endogenous peptide hormone, works through inhibition of bone resorption by binding to osteoclasts through a high-affinity receptor. However, the reduction in bone turnover associated with calcitonin treatment is much smaller than that seen with other antiresorp¬tive agents [18]. Calcitonin also seems to have an analgesic effect on acute painful vertebral fractures [19].
vi Denosumab
Denosumab, a new antiresorptive agent, is a fully human monoclonal antibody that inhibits RANKL, a protein required for osteoclast formation, function and survival [20]. On the basis of this evidence of antifracture efficacy, denosumab appears to be another promising first-line osteoporosis therapy, although (as with any new agent) long-term safety data are required to establish its role in patient care [21].
II Osteoblast Activators
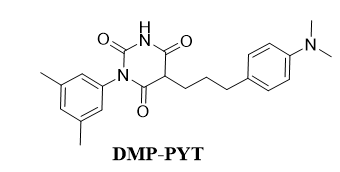
i DMP-PYT
Small molecules were isolated that activated both SMADs and β-catenin, that led to the discovery of a novel potent osteogenic compound, DMP-PYT. They worked through BMP2 stimulation, DMP-PYT substantially increased osteoblast differentiation through enhanced expression of osteoblast-specific genes and accelerated calcification featured by activation of BMPs expression [22]. DMP-PYT promoted BMP2-induced SMAD1/5/8 phosphorylation and β-catenin expression, the latter in a BMP2-independent manner. DMP-PYT alone enhanced nuclear localization of β-catenin to promote the DNA-binding and transcriptional activity of T-cell factor, thereby resulting in increased osteoblast differentiation in the absence of BMP2 [22].
ii Nuclear Factor-Kappa B (RANK) Signaling
Bone remodeling involves osteoclast activation, resorption, and reversal, prior to osteoblast migration into the bone pit. The Receptor Activator of NF-kB (RANK) signaling pathway plays an important role in bone remodeling. Two components of the RANK signaling pathway, RANK Ligand (RANKL) and the decoy receptor Osteoproteger in (OPG), are expressed predominantly on the surface of osteoblasts, while RANK is principally expressed on the surface of osteoclasts [23].
iii Adrenomedullin
Adrenomedullin is a potent stimulator of osteoblastic activity in vitro and in vivo. Adrenomedullin is a 52-amino acid vasodilator peptide produced in many tissues, including bone. It has 20% sequence identity with amylin, a regulator of osteoblast growth, and circulates in picomolar concentrations. Adrenomedullin also increased [3H] thymidine incorporation into cultured neonatal mouse calvaria. Adrenomedullin stimulated phenylalanine incorporation into both isolated osteoblasts and calvaria [24].
Article Info
Article Type
Review ArticlePublication history
Received: Mon 25, Nov 2019Accepted: Fri 20, Dec 2019
Published: Mon 30, Dec 2019
Copyright
© 2023 El-Shimaa M. N. Abdelhafez. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ORD.2019.01.05
Author Info
Adel M. Abdel-Hakem El-Shimaa M. N. Abdelhafez Salsabel M. N. A. Ali Sara Mohamed Naguib Abdelhafez
Corresponding Author
El-Shimaa M. N. AbdelhafezDepartment of Medicinal Chemistry, Faculty of Pharmacy, Minia University, Minia, Egypt
Figures & Tables
References
- Wang Z, McCauley LK (2011) Osteoclasts and odontoclasts: signaling pathways to development and disease. Oral Dis 17: 129-142. [Crossref]
- Chu TMG, Liu SSY, Babler WJ (2014) Craniofacial biology, orthodontics, and implants. Elsevier Basic and Applied Bone Biology 225-242.
- Zubery Y, Dunstan CR, Story BM, Kesavalu L, Ebersole JL et al. (1998) Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect Immun 66: 4158-4162. [Crossref]
- Chen JS, Sambrook PN (2011) Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol 8: 81-91. [Crossref]
- Gao B, Chen W, Hao L, Zhu G, Feng S et al. (2013) Inhibiting periapical lesions through AAV-RNAi silencing of cathepsin K. J Dent Res 92: 180-186. [Crossref]
- Montoya Carralero JM, Parra Mino P, Ramírez Fernández P, Morata Murcia IM, Mompeán Gambín Mdel C et al. (2010) Dental implants in patients treated with oral bisphosphonates: a bibliographic review. Med Oral Patol Oral Cir Bucal 15: e65-e69. [Crossref]
- Serra M, Llorca CS, Donat FJ (2008) Oral implants in patients receiving bisphosphonates: a review and update. Med Oral Patol Oral Cir Bucal 13: E755-E760. [Crossref]
- Suzuki J, Scher E, Ucer C, Lee C (2013) Dental management of patients receiving antiresorptive therapy. IDT.
- Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19: 733-759. [Crossref]
- Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin Proc 83: 1032-1045. [Crossref]
- Kalra S, Jain V (2013) Dental complications and management of patients on bisphosphonate therapy: A review article. J Oral Biol Craniofac Res 3: 25-30. [Crossref]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA et al. (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291: 1701-1712. [Crossref]
- Torgerson DJ, Bell Syer SEM (2001) Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trials. BMC Musculoskelet Disord 2: 7. [Crossref]
- Barrett Connor E, Mosca L, Collins P, Geiger MJ, Grady D et al. (2006) Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New Engl J Med 355: 125-137. [Crossref]
- Adomaityte J, Farooq M, Qayyum R (2008) Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost 99: 338-342. [Crossref]
- Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S et al. (2010) Lasofoxifene in postmenopausal women with osteoporosis. New Engl J Med 362: 686-696. [Crossref]
- Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR et al. (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3‐year, randomized, placebo‐, and active‐controlled clinical trial. J Bone Miner Res 23: 1923-1934. [Crossref]
- Chesnut CH, Azria M, Silverman S, Engelhardt M, Olson M et al. (2008) Salmon calcitonin: a review of current and future therapeutic indications. Osteoporos Int 19: 479-491. [Crossref]
- Silverman S, Azria M (2002) The analgesic role of calcitonin following osteoporotic fracture. Osteoporos Int 13: 858-867. [Crossref]
- Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B et al. (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43: 222-229. [Crossref]
- Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New Engl J Med 361: 756-765. [Crossref]
- Bae SJ, Kim HJ, Won HY, Min YK, Hwang ES (2017) Acceleration of osteoblast differentiation by a novel osteogenic compound, DMP-PYT, through activation of both the BMP and Wnt pathways. Scient Rep 7: 8455.
- Golden D, Saria EA, Hansen MF (2015) Regulation of osteoblast migration involving receptor activator of nuclear factor‐kappa B (RANK) signaling. J Cell Physiol 230: 2951-2960. [Crossref]
- Cornish J, Callon KE, Coy DH, Jiang NY, Xiao L et al. (1997) Adrenomedullin is a potent stimulator of osteoblastic activity in vitro and in vivo. Am J Physiol 273: E1113-E1120. [Crossref]
