Complete Thoracic Ectopia Cordis, Dilemma of the Outcome: A Case Report
A B S T R A C T
Background: Ectopia Cordis (EC) is a rare congenital condition where the heart is partially or completely lies outside the thoracic cavity (extrathoracic), uncovered by pericardium and skin. Many works of literature reported EC is a rare congenital abnormality with an incidence of about 5-8 per 1 million live births and includes about 0.1% of congenital heart diseases.
Methods: This was a male baby, weighing 2.900 kg, received with cyanosis with a defect in the anterior chest wall and heart protruding out through it. On initial physical examination, split sternum with complete thoracic EC (beating outside the thoracic cavity with a complete absence of the pericardium with the apex pointing upwards) were reported.
Results: The baby’s poor general condition did not allow further radiologic studies and echocardiography could not be performed. By the time, an arrangement had been planned for him to undergo cardiothoracic referral; unfortunately, he ran a downhill course and succumbed within 36 hours of life.
Conclusion: The important for submitting such findings will assist our team: pediatric surgeons, obstetricians, pediatricians and, neonatal intensivists to develop future management strategies when they are enrolled or confronted with such cases, by improving the outcome through a precise workup design to provide the optimal evaluation, diagnosis, and management roadmaps of potential cases of EC.
Keywords
Ectopia cordis, complete, outcome, dilemma
Introduction
Ectopia Cordis (EC) is a rare congenital condition where the heart is partially or completely lies outside the thoracic cavity (extrathoracic), uncovered by pericardium and skin [1]. Many works of literature reported EC is a rare congenital abnormality with an incidence of about 5-8 per 1 million live births and includes about 0.1% of congenital heart diseases [2]. Embryologically and through the intrauterine fetal life, it occurs because of the failure of maturation of the midline mesodermal components of the chest and abdomen. It may occur as an isolated malformation or it may be associated with body wall defects that affect the thorax, abdomen, or both [3]. Many controversies about the management outcome, some authors reported the prognosis is poor, depends on the associated defects, others submitted, with the advances in the medical and surgical resources more patients, have been successfully treated and have survived [4]. We reported a case of EC managed initially in our neonatal care and pediatric surgery units.
Case Report
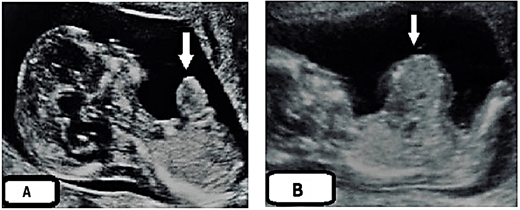
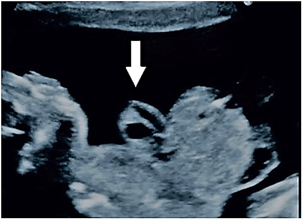
This was a male baby, weighing 2.900 kg, a product of elective cesarean section to a 34-year-old woman (known consanguinity), gravida 4 paras 1, had no regular prenatal care through her gestation and uneventful pregnancy during her first ultra-sonographic (US) study. There was no family history of congenital abnormalities. No clear hint about her viral and toxoplasmosis screening despite her previous history of abortions. Routine re US assessment at 24 weeks of her gestation announced fetus with anterior wall defect (minor omphalocele), reevaluation suggested at 28 weeks of gestation wrote the same image (Figures 1A & 1B). Surprisingly, the second-hand opinion submitted the same previous images (omphalocele containing part of the small bowel) (Figure 2).
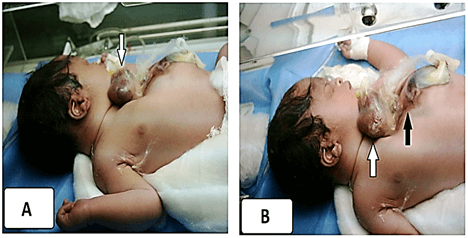
Accordingly, the obstetrician physician assigned the elective cesarean for her at 37 weeks of gestation. The baby was received with cyanosis with a defect in the anterior chest wall and heart protruding out through it. The newborn was scored 7 (1 min) and 6 (5 min) on the Apgar scale with hypotonia, weak cry, and generalized facial cyanosis. On initial physical examination, split sternum with complete thoracic EC (beating outside the thoracic cavity with a complete absence of the pericardium with the apex pointing upwards) were reported (Figures 3A & 3B).
Figure 1: A) US assessment to the mother at 24 weeks of her gestation announced fetus with anterior wall defect (minor omphalocele). arrowed. B) Re-evaluation by US suggested at 28 weeks of gestation reported the same image with (A) abdominal wall defect, arrowed.
Figure 2: Second-hand opinion US study at 30 weeks of the mother gestation submitted the same previous reports (omphalocele containing part of the small bowel) instead of well obvious ectopic heart and well announced chambers, arrowed.
Figure 3: A) Split sternum with complete thoracic EC (beating outside the thoracic cavity with a complete absence of the pericardium with the apex pointing upwards), white arrow. B) The lower part of the sternum and upper abdominal wall was also deficient without a protruding mass or viscera, black arrow.
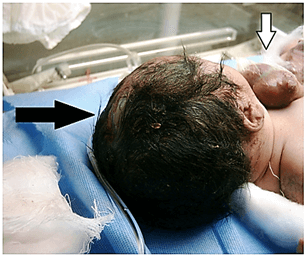
The lower part of the sternum and upper abdominal wall was also deficient without a protruding mass or viscera (the sternum was not visualized). A defect 5-6 cm diameter, in the mid bony skull and the scalp tissue through the metopic suture and anterior fontanelle (Cranium Bifidum) with mild protrusion of the dura (encephalocele) (Figure 4). Initial workup was planned, the baby was looking sick, lethargic, hypothermic, tachypneic, having poor perfusion and central cyanosis, and had shallow breathing, he had severe sternal retraction on inspiration. The heart rate was 154/min and the peripheral pulses were feeble. The baby approached by stabilization, warmer care with a strict aseptic measures, O2 supplementation, intravenous fluid administration, and nasogastric decompression although the enteral feeding was not commenced. The infant’s heart was covered with a warm saline-soaked sterile dressing and broad-spectrum antibiotics were initiated. The first blood profiles revealed; haemoglobin 14.4 gm/dl, packed cell volume was 48%, initial blood sugar, urea, and electrolytes were normal. A portable abdominal and chest x-ray done for him and revealed a complete bifid sternum, wide separation of the sternal ends of the clavicles and widening of the superior mediastinum and. abnormal cardiac position (exposure and pixel of the image not eligible for publishing). The baby poor general condition did not allow further radiologic studies and echocardiography could not be performed. By the time, an arrangement had been planned for him to undergo cardiothoracic referral; unfortunately, he ran a downhill course and succumbed within 36 hours of life. The parents declined the postmortem newborn’s autopsy. The parents were counseled about the genetic study and they neglected this opinion.
Figure 4: A defect in the mid bony skull and the scalp tissue through the metopic suture and anterior fontanelle (Cranium Bifidum) with mild protrusion of the dura (encephalocele), black arrow.
Discussion
Ectopia Cordis is a very rare malformation syndrome. Many theories were announced in their attempt to explain this anomaly, including the amniotic band theory, the vascular disruption theory, the theory of a defect in the fetal folding process, and the theory of disturbances of field development [4, 5]. Developmental fields are those units of the embryo in which the development of a particular complex structure is determined and controlled in a coordinated, temporally synchronous, and hierarchical manner [5, 6]. While EC is generally considered an isolated, sporadic malformation, there have been a number of reports linking it to chromosomal abnormalities, including trisomy 18, Turner syndrome, and 46, XX, 17q+ [7]. Chromosomal analysis is indicated in a patient with prenatally diagnosed especially if other anomalies are also identified. Clinically, it has been classified into five types according to the cardiac location: cervical, cervicothoracic, thoracic (the most common one and represented about 60% of cases), thoracoabdominal and abdominal [7]. It is frequently associated with other congenital defects involving multiple organ systems. Ventricular septal defects and tetralogy of Fallot are the most common associated intracardiac defects [7]. EC is commonly associated with an anterior diaphragmatic hernia, while omphalocele or gastroschisis are the most common associated abdominal wall defects. Our case seemed to be the thoracic type. The following characteristics were reported in our patient; completely bifid sternum, anterior extrathoracic heart with an absence of the parietal pericardium [7, 8]. The various clinical types of EC have different prognoses. Cervical and thoracic EC is usually fatal within days because the heart is exposed and malformed. This defect may also be associated with other congenital anomalies like anencephaly, hydrocephaly, cranial, neural tube defects, and facial malformations including cleft lip or palate, genitourinary or gastrointestinal malformation [9].
In our patient, well obvious bifid cranium was reported. EC can be diagnosed during pregnancy by the US; an early plan should be made for elective cesarean section. The use of a three or the four-dimensional US and its combination with Doppler allows for a more accurate early diagnosis. Magnetic resonance imaging (MRI) is also becoming commonplace in prenatal evaluation to document and plan for the management of complicated congenital anomalies [10]. The qualification of the US study in our patient with the limited experiences despite the two hand opinions and institutional resources did not allow the team to draw the perfect road map of the expected workup that should be assigned for such patients. Such a dilemma made this pitfall in the diagnosis of the severity of the pathology and the estimation of the precise outcome. Specific management of EC is the repair of the intracardiac defect, placement of the heart into the thorax, repair of the associated abdominal anomaly, then closure of the chest wall defect. The first attempted repair of EC was performed in 1925 by Cutler and Wilens [11]. In 1975 Koop achieved the first successful repair in two stages [12, 13]. Amato et al. reported a successful single-stage repair of thoracic EC in 1995 [14]. During surgical closure, in most cases, the thoracic cavity is small with little mediastinal space for the heart. In works of literature, In spite of surgical treatments, few patients with EC survive and most of them die in the first few hours, days, or weeks of life [14]. The prognosis of this condition is usually poor; some authors reported that termination of pregnancy prior to viability should be considered when it diagnosed during the antenatal visit [15]. Because of the short time post-delivery, we could not claim a decision strategy to salvage our patient and all resuscitation measurements did not have an impact on the previously determined poor outcome.
Conclusion
We highlighted a complete thoracic EC case was extremely rare, with complex mechanisms leading to a large spectrum of clinical manifestations ranging from EC itself and other associated cranial anomalies. The importance of submitting such findings will assist our team: pediatric surgeons, obstetricians, pediatricians and neonatal intensivists to develop future management strategies when they are enrolled or confronted with such cases, by improving the outcome through a precise workup design to provide the optimal evaluation, diagnosis, and management roadmaps of potential cases of EC. The prognosis of EC is largely related to the severity and the complexity of this anomaly.
Ethics Statement
The study conformed to the guidelines of the institutional review board of our Institution, which approved its ethical aspects.
Consent
Written informed consent was obtained from the patient’s parent who participated and managed in this report for publication and any accompanying images.
Acknowledgement
The author expresses sincere gratitude to all the pediatric surgery unit staff, pediatric outpatient's clinic, at the Maternity and Child Teaching Hospital, Al Qadisiya, Iraq, for their assistance.
Conflicts of Interest
None.
Funding
None.
Author Contributions
Each author contributed equally to the drafting and review of the manuscript.
Article Info
Article Type
Case ReportPublication history
Received: Mon 15, Mar 2021Accepted: Thu 25, Mar 2021
Published: Fri 09, Apr 2021
Copyright
© 2023 Mohammed J Aboud. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.04.05
Author Info
Mohammed J Aboud Noor M Abudi Haidar M Joudi Zeena M Joudi
Corresponding Author
Mohammed J AboudPediatric Surgery Unit, The Maternity and Child Teaching Hospital, Al Diwaniya, Iraq
Figures & Tables
References
1. Alphonso N, Venugopal PS, Deshpande R, Anderson D (2003) Complete thoracic ectopia cordis. Eur J Cardiothorac Surg 23: 426-428. [Crossref]
2. Donizete Gonçalves F, Rotatori Novaes F, Alves Maia M, De Assis Barros F (2007) Thoracic ectopia cordis with anatomically normal heart. Rev Bras Cir Cardiovasc 22: 245-247. [Crossref]
3. Cabrera A, Rodrigo D, Luis MT, Pastor E, Galdeano JM et al. (2002) Ectopia cordis and cardiac anomalies. Rev Esp Cardiol 55: 1209-1212. [Crossref]
4. Morales MJ, Patel SG, Duff JA, Villareal RL, Simpson JW (2000) Ectopia cordis and other midline defects. Ann Thorac Surg 70: 111-114. [Crossref]
5. Thomas WS (2010) The embryologic origin of ventral body wall defects. Semin Pediatr Surg 19: 209- 214. [Crossref]
6. Humpl T, Huggan P, Hornberger LK, McCrundle BW (1999) Presentation and outcomes of ectopia cordis. Can J Cardiol 15: 1353-1357. [Crossref]
7. Pamidi N, Vollala VR, Nayak S, Bhat S (2008) Ectopia cordis and amniotic band syndrome. Arch Med Sci 4: 208-211.
8. Khoury MJ, Cordero JF, Rasmussen S (1988) Ectopia cordis, midline defects and chromosome abnormalities: an epidemiologic perspective. Am J Med Genet 30: 811-817. [Crossref]
9. Bick D, Markowitz RI, Horwich A (1988) Trisomy 18 associated with ectopia cordis and occipital meningocele. Am J Med Genet 30: 805-810. [Crossref]
10. Garson A, Kawkins EP, Mullins CE, Edwards SB, Sabiston DC et al. (1978) Thoracoabdominal ectopia cordis with mosaic Turner’s syndrome. Report of a case. Pediatrics 62: 218-228. [Crossref]
11. Tongsong T, Wanapirak C, Sirivatanapa P, Wongtrangan S (1999) Prenatal sonographic diagnosis of ectopia cordis. J Clin Ultrasound 27: 440-455. [Crossref]
12. Amato JJ, Dughlous WI, Desai U, Berke S (2000) Ectopia cordis. Chest Surg Clin N Am 10: 297-316. [Crossref]
13. Dobell AR, William HBU, Long RW (1982) Staged repair of ectopia cordis. J Pediatr Surg 17: 353-358. [Crossref]
14. Amato JJ, Jonathan Z, Talwalkar NG (1995) Single stage repair of thoracic ectopia cordis. Ann Thorac Surg 59: 518-520. [Crossref]
15. Homberger LK, Colan SD, Lock JE, Wessel DL, Mayer JE (1996) Outcome of patients with ectopia cordis and significant intracardiac defects. Circulation 94: 32-37. [Crossref]
