Clinical Case of Pregnancy and Follow-Up of Bartter Syndrome (Type II) with a Novel Mutation
A B S T R A C T
Background: Bartter syndrome is a rare autosomal recessive inherited salt wasting tubulopathy, it`s incidence proportion is 1.2 cases per 1.000.000 live births. The present case - report discusses a clinical case of an antenatal Bartter syndrome (type II) with a novel mutation and it`s course from antenatal presentation to 6 months postpartum.
Case Presentation: The case-report discusses a clinical case of an antenatal Bartter syndrome (type II) with a novel homozygous missense variant mutation in KCNJ1 gene: c.554C>T (p. Pro185Leu). Symptoms presented from 24 weeks of pregnancy as premature labour threats, maternal dyspnoea and severe polyhydramnios (amniotic fluid index 36 cm). Therapeutic interventions included use of indomethacin, dexamethasone, micronized progesterone and three consequent amnioreductions. Pregnancy was prolonged until 32 weeks and induced due to severe reoccurring polyhydramnios, progressing maternal dyspnoea and inability to perform next amnioreduction. Labour was complicated by severe placental abruption and new born – boy was referred to neonatal intensive care unit. Neonatal period was complicated by electrolyte abnormalities: hyponatremia, hypochloremic metabolic alkalosis, transient hyperkalaemia that gradually developed into hypokalaemia, hypercalcemia and elevated rennin and aldosterone levels characteristic to type II Bartter syndrome. At 6 months (corrected age 4 months) he is gaining weight within normal ranges and his psychomotor development is ahead of his corrected age, without any need for daily medications.
Conclusion: The present case report describes the clinical course of a Bartter syndrome is of high importance, due to the reason that it shows clinical course of patient with novel mutation and offers one of the ways how to manage the disease. The described novel mutation may have favourable prognosis for neonate. The pregnancy should be managed as high-risk pregnancy with expertise in perinatal diagnostics and interventions. Early recognition, and interventions, are and essential to prolong a pregnancy and lessen prematurity complications.
Keywords
Neonatal Bartter syndrome, Type II Bartter syndrome, novel mutation, amnioreduction, case report, chorio-amniotic separation
Case Study
I Pregnancy
29 years old pregnant woman, obese with body mass index (BMI) 46 kg/m2, was referred to second opinion ultrasound to perinatal center because of severe polyhydramnion at 26+4 weeks of gestation . Previously the patient underwent standard regular antenatal care in outpatient care services. Her pregnancy was spontaneous; she was expecting a baby of non-consanguineous marriage. As to the ethnic origin, woman and her husband were Latvians. The pregnancy was complicated by a subchorionic hematoma with bleeding at early 10/11 weeks, respiratory virus infection at 17 weeks and flu infection at 24 weeks of gestation. All other standard follow-ups were unremarkable.
Patient’s complaints on admission included shortness of breath, increased vaginal discharge and lower abdominal pain. Accelerated abdominal growth and increased vaginal discharge during the last two-week period were reported. A detailed ultrasound examination revealed anatomically normal foetus. The fetal biometric data of the patient was consistent with 27/28 gestational weeks (weight 1007g) and amniotic fluid index, appeared to be 36.4 cm, showing severe polyhydramnios, ultrasound also demonstrated a shortened cervix (7 mm). A normal oral glucose tolerance test helped the researchers to rule out gestational diabetes mellitus. A magnetic resonance examination allowed to exclude severe malformations including tracheal agenesis (Table 1).
Table 1: Common causes for polyhydramnios [1, 2].
|
Structural abnormalities (Impaired Swallowing) |
|
Gastrointestinal obstruction: Digestive tract atresia; Diaphragmatic hernia; Tracheal atresia; Trachea oesophageal fistula; Thoracic mass; Neuro-muscular : Myotonic dystrophy Arthrogriposis Intracranial anomaly Fetal akinesia/hypokinesia sequence Caniofacial : Cleft lip/palate Micrognathia Neck mass |
|
Structural abnormalities (Excess Urine Production) |
|
Ureteropelvic obstruction Mesoblastic nephroma Cardiac structural anomaly Tachyarrythmias Sacrococcygeal teratoma Hydrops |
|
Maternal–fetal infection Cytomegalovirus Toxoplasmosis Herpes simplex infection Parvovirus infection |
|
Aneuploidies, sex chromosome aneuploidies |
|
Maternal diabetes |
|
Placental tumours |
|
Bartter syndrome |
Dexamethasone 6 mg i/m every 12 h for 48 h (for respiratory distress prophylaxis) and micronized progesterone vaginally (for a short cervix) 200 mg once a day were administered. Later, diagnostic amniocentesis and amnioreduction were carried out to facilitate maternal dyspnoea and exclude chromosomal abnormalities and TORCH infections. Herpes simplex, toxoplasmosma, cytomegalovirus, ebstein barr virus infections were excluded using real time polymerase chain reactions (PCR-RT). The results are shown in (Table 2).
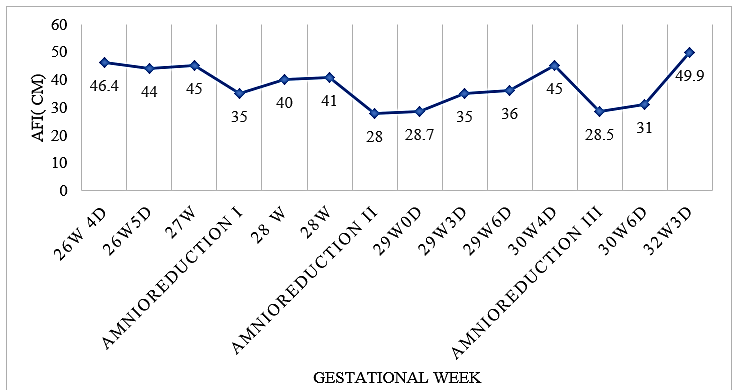
The amniotic fluid analysis results showed low protein level (1.96 g/l); elevated sodium (144 mmol/l) and chlorides in upper reference limit (108 mmol/l). Results of severe polyhydramnion with low protein and without presence of infections or severe malformations enabled the researchers to suggest that there is a presence of antenatal Bartter syndrome. For this reason, a therapy with indomethacin 50 mg p/r 4x day at 28 weeks of gestation was prescribed (for a week). However, severe polyhydramnion was reoccurring and two more amnioreductions were needed (Graph 1) at 28 and at 30 weeks of gestation.
Graph 1: Amniotic fluid index through amnioreductions.
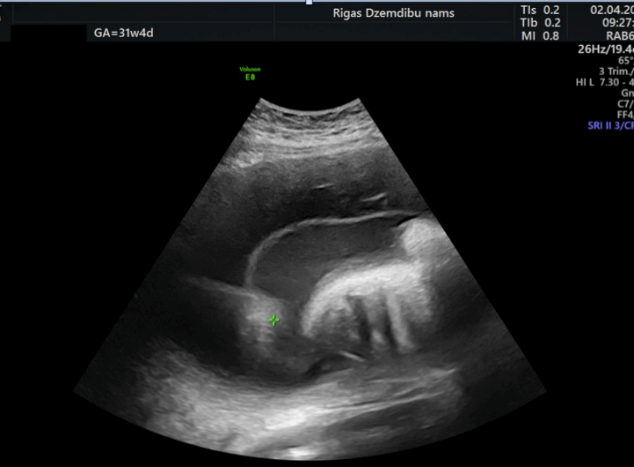
At 31 weeks of gestation a wide chorioamniotic separation (CAS) on the anterior uterine wall was detected on the image taken (Figure 1). At 32+2 weeks of gestation a decision to induce labour was made because maternal dyspnoea and inability to preform fourth amnioreduction due to wide chorioamniotic separation. Amniotomy was used for induction and 3.5 l of clear amniotic fluid was drained. Regular uterine activity developed, and the patient progressed normally during the labour until a cervical dilatation reached 8-9 cm, when a severe placental abruption occurred. An emergency Caesarean section was performed, and massive postoperative atonic haemorrhage developed during the surgery. The total blood loss was 3.5 litres. Bleeding was managed with oxytocin, misoprostol, Bacri baloon tamponade, fluid resuscitation and haemotransfusion. The postoperative period was uneventful. Furthermore, a neonate was delivered, using podalic extraction method. The new-born was transferred to the Neonatal Intensive Care Unit (NICU) with Apgar score 7/7.
Figure 1: Chorioamniotic separation.
II Newborn
The neonate weighed 1840 g (50 percentile) and had Apgar scores of 7-7. A clinical evaluation of the new-born showed subcutaneous tissue oedema, respiratory difficulties, hypotonia and hyporeflexia. Due to this condition he received an initial stabilization, tactile stimulation and airway clearance and respiratory support with peak inspiratory pressure of 5 cm H2O. Moreover, NaCl 0.9 % 20 ml was administered (as a bolus) in order to maintain circulation. At the NICU, where the infant was transferred to, further examination revealed hematoma on the right foot and leg. Active bleeding, however, was not detected. His blood pressure was 52/26 mmHg; the respiratory rate was 60 x/min. According to the Silverman-Andersen Retraction scoring system, the infant’s score was 3-4 points. Therefore, respiratory support with continuous positive airway pressure was started.
From the first days of his life electrolyte abnormalities developed: hyperkalemia up to 8.1 mmol/l (3.6-6.1 mmol/l) which gradually decreased and later developed into hypokalemia 2.78 mmol/L (Graph 2). From the 5th day of the now-borns life hypochloremia 91.8mmol/L (95-116 mmol/l) and hypercalcemia 2.7 mmol/l (1.9-2.6 mmol/l) developed. In addition, ionized calcium levels arose on the 2nd week of his life. At the age of two weeks transient hyponatraemia 120.2 mmol/l (132-147 mmol/l) developed. After the discharge from the Maternity Hospital, serum calcium levels remain elevated: at corrected age 4 months the level is 2.72 mmol/L, two months later 2.90 mmol/L.
Simultaneously there was metabolic alkalosis pH 7.424 (7.2-7.4), hCO3-act 29.0 mmol/L (18.6-22.6 mmol/l) and an increased anion gap 33.9 mmol/L (10-20 mmol/l), thought to be due to hypochloremia. Severe polyuria with dehydration was not seen and neonates condition gradually improved. Next diagnostic steps included renin and aldosterone analysis, it showed hyperreninemia (>500 µU/ml) and hyperaldosteronism (>1000 pg/ml). From the 5th day renal ultrasound suggested early nephrocalcinosis.
Graph 2: Neonate potassium level.
As on the 2nd day of his life the infant developed neonatal apnoea of prematurity, oral caffeine was added to the therapy. The infant was in an incubator; parenteral feeding from the first day was carried out. Mother’s breast milk of 17 ml every 3 hours was given. K vitamin of 1 mg was administrated i/v on the 1st and 4th day of life. From the first day of infant’s life, antibacterial, including Ampicillin 92 mg together with Bifidobacterium was prescribed as per protocol in NICU.
In addition, electrolyte supplementation therapy which implied the use of NaCl 0.9%, Calcium gluconate 8.9 mg/ml and NaCl 5.85% administered twice a day, was performed. Also, the infant underwent phototherapy as a treatment of hyperbilirubinemia. On the 5th day the infant’s condition improved. From that point breastfeeding was successfully started, periodically supplemented with formula. The genetic evaluation revealed homozygous mutation in KCNJ1 gene, c.554C>T(Pro185Leu) confirming Bartter syndrome (type II). In 39 days, he was discharged from Children’s hospital. At the time he weighed 2660 g; his length was 48 cm.
III Follow-Up
At 6 months (corrected age: 4 months) the baby weights 5.6 kg and is 68 cm tall. His motor development is ahead of his corrected age. As concerns the baby’s motor skills, they are symmetric. He can roll on his both sides and stomach, hold his head and chest upright, lying on his stomach, differentiate people and make hand-hand and hand-mouth contact. There`s no need for daily medication and he is observed by paediatrician and nephrologist. Renal ultrasound shows bilateral nephrocalcinosis without negative dynamic.
Discussion and Conclusion
The present case deals with novel homozygous missense mutation detected by Bartter syndrome gene in KCNJ1 gene: c.554C>T (p. Pro185Leu). Both parents of the infant appeared to be sequencing the carriers for the above-mentioned type of mutation. No genetic disorders or infant deaths were noted in family tree. After the conduction of literature review it was concluded that the present case of Barter syndrome (type II) is the first one illustrated in medical literature, described with this specific mutation. The value of this report lies in that it can give in insights into a clinical course for parents and doctors who may encounter this genotype in future.
In this specific case the severe manifestation of the disease occurred during pregnancy. Notable that its manifestation in the neonatal period was milder than expected, which means there was no severe dehydration and need to take medication on the daily basis. The infant’s development was normal up to 6 months of age. Bartter syndrome (type II), defined also as antenatal syndrome, is caused by a mutation in gene KCNJ1 located in 11q24, leading to dysfunction in renal outer medullary potassium channel (ROMK) in thick ascending Henles loop [3]. As it was stated in other research articles, the clinical evidences of the disease may be electrolyte disbalance (hyponatraemia, hypochloremia, transient hyperkalemia, hypokalaemia that develops during time span and hypomagnesemia), metabolic alkalosis, polyuria, hyperreninaemic hyperaldosteronism, nephrocalcinosis and failure to thrive in infancy [4]. Prenatally Bartter syndrome (type II) manifests with severe polyhydramnion from 23-26 gestational weeks and premature delivery [5].
The neonates with Bartter syndrome (type II) are typically born from 31 up to 35 weeks, with a vast majority being premature at approximately 33 weeks [6]. Hypercalcemia, as seen in this case, is not a typical finding in antenatal Bartter syndrome. Atypical presentation with hypercalcemia due to hyperparathyroidism in type I Bartter syndrome have been previously reported in literature [7]. Unfortunately, we have no data of parathyroid hormone tests in this case. In the present case Bartter syndrome was preliminary diagnosed by excluding the common causes of severe polyhydramnion (Table 2) with fetal karyotype, magnetic resonance imaging and by determining low protein in amniotic fluid. Low protein is thought to be due to the dilution, but not the excess of fetal proteinuria [8]. Amniotic fluid sodium was slightly elevated, and the level of chlorides appeared to be in the upper reference limit. According to Garnier (2010), electrolytes should not be used in diagnosis. In previous researches Bartter index has been proposed for diagnostics, but it should be taken into account that it has low sensitivity and specificity, which approximately 85.7% and 84.2% [9]. The diagnosis of the disease should be considered when there is a presence of severe reoccurring polyhydramnios with no morphological anomalies from the second trimester with low protein in amniotic fluid biochemistry.
Table 2: Amniotic fluid analysis.
|
Tested infection |
Results |
Reference values (Garnier et. al 2010, Nature) |
Reference values (Bernie et al. Am J Obstet Gynecol 1974) |
|
Herpes simplex DNA PCR-RT |
negative |
||
|
Toxoplasma gondii DNA PCR-RT |
Negative |
||
|
Cytomegalovirus DNA PCR-RT |
Negative |
||
|
Ebstein Barr virus DNA PCR-RT |
negative |
||
|
Amniotic fluid biochemistry |
|
||
|
Protein |
1.96 g/l |
5.2 (2.3–9.9) g/l |
|
|
Potassium (K+) |
3.6 mmol/l |
3.8 (3.1–4.6) mmol/l |
4.0 ±0.2 mmol/l |
|
Sodium (Na+) |
144 mmol/l |
136 (129–141) mmol/l |
136.0 ± 6.8 mmol/l |
|
Chloride (Cl-) |
108 mmol/l |
111 (105–118) mol/l |
108.8 ±3.2 mmol/l |
|
Calcium (Ca2+) |
1.41 mmol/l |
1.47 (0.67–1.93) mmol/l |
7.4 ±2.2 (mg/dl) |
|
Karyotype |
46, XY |
|
|
During the pregnancy indomethacin treatment was started from 28-29 weeks. It is thought that indomethacin stimulates fetal secretion of arginine vasopressin and inhibits prostaglandin synthetase leading to decreased diuresis, renal salt wasting and polyhydramnios [1]. The suggested maximal dose is 2-3mg kg/day [3]. There are several reports of indomethacin use in neonatal period, but less during pregnancy. There are no randomised trials for its use due to the rarity of the syndrome. And Society for Maternal-Fetal Medicine advices against its use in the cases if it is needed to decrease AFI. Caution should be taken against early ductus arteriosus construction and it should be used until 32 weeks of gestation [3].
Amnioreduction, which is considered to be a safe procedure in singleton pregnancies, was performed to reduce severe polyhydramnion, defined as AFI ≥35 cm , and maternal dyspnoea [1, 10]. Other similar case reports claim that it is commonly used regiment for antenatal Bartter syndrome management [3, 11-13]. Diagnostic problems were encountered in the present case as the patient had the 3rd stage of obesity. Punctures were technically difficult due to problems visualising the needle tips precise location. According to literature, obesity with BMI > 40 kg/m2 is linked to multiple needle insertions and more frequent fetal losses after amniocentesis [14]. Chorioamniotic separation formation (CAS) is linked to amniocentesis, amnioreduction and fetal surgery [10, 15]. Forth amnioreduction weren`t carried out due to case reports of complete CAS and fetal demise in utero after amniocentesis preformed on pre-existing chorioamniotic separation [15].
However, in the present case the difficulties with diagnosing were not the only ones. The delivery of the infant was technically challenging as well, as first category caesarean section was performed due to a placental abruption. As a result, the neonate was delivered by a podalic extraction, resulting in a small hematoma on the right foot and leg. The infant had persistent hypochloremia metabolic alkalosis, hyperkalaemia, which gradually developed into hypokalaemia, hypercalcemia, one-time hyponatraemia, hyperreninemia and hyperaldosteronism. Neonatal complications may arise from untreated severe dehydration, which may lead to severe electrolyte imbalances and arrhythmias. Gallstones, growth retardation and decrease in bone mineral density have been described in other types of Bartter syndrome [16, 17].
Coming to the conclusions it should be stated that if to manage Bartter syndrome (type II) adequately, it has a positive prognosis. Typically, infant’s somatic, neurodevelopmental growth is normal and renal failure is uncommon [17]. For this reason, it is important to avoid associated complications to improve an outcome. In the case of prenatal Bartter syndrome, early recognition, and interventions, such as amnioreductions, is safe and essential to prolong a pregnancy and lessen prematurity complications. At the same time, it should be balanced, as mother’s wellbeing should be taken into consideration.
To summarise, the suggestion of the authors of this paper are the following:
i. Type II Bartter syndrome with described novel mutation may have favourable prognosis for neonate. The pregnancy should be managed as high-risk pregnancy with expertise in perinatal diagnostics and interventions. If a woman with risk factors such as severe polyhydramnios, high body mass index and serial amnioreductions is in labour, cross matched blood must be available due to the high risk of placental abruption and postpartum haemorrhage.
ii. From women’s perspective she is thankful for all doctors who took care for her and is thankful that her son is thriving well.
Consent and Ethical Approval
Written informed consent for case study publication was obtained from the women. Riga Stradins university ethics approval was granted.
Competing Interest
None.
Funding
None.
Availability of Data and Materials
The data that support the findings of this study are available on request from the corresponding author LL. The data are not publicly available due to that could compromise research participant privacy and consent.
Author Contributions
LL and SK were involved in manuscript preparation and literature review. DR, NV, AM and GJ were involved in patient care in obstetric unit. KR is involved in ongoing neonate postnatal follow up. The authors have read and approved final manuscript.
Acknowledgement
Not applicable.
Abbreviation:
BMI: Body mass index
AFI: Amniotic fluid index
PCR- RT: Polymerase chain reaction real time
DNA: Deoxyribonucleic acid
CAS: Chorioamniotic separation
NICU: Neonatal Intensive Care Unit
ROMK: Renal outer medullary potassium channel
Article Info
Article Type
Case ReportPublication history
Received: Wed 29, Apr 2020Accepted: Tue 12, May 2020
Published: Mon 18, May 2020
Copyright
© 2023 Laura Lūse. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CROGR.2020.01.05
Author Info
Anna Miskova Dace Rezeberga Gita Jansone Kristīne Rasnača Laura Lūse Natālija Vedmedovska Sabīne Kovale
Corresponding Author
Laura LūseDepartment of Obstetrics and Gynecology, Riga Stradins University, Riga, Latvia
Figures & Tables
Table 1: Common causes for polyhydramnios [1, 2].
|
Structural abnormalities (Impaired Swallowing) |
|
Gastrointestinal obstruction: Digestive tract atresia; Diaphragmatic hernia; Tracheal atresia; Trachea oesophageal fistula; Thoracic mass; Neuro-muscular : Myotonic dystrophy Arthrogriposis Intracranial anomaly Fetal akinesia/hypokinesia sequence Caniofacial : Cleft lip/palate Micrognathia Neck mass |
|
Structural abnormalities (Excess Urine Production) |
|
Ureteropelvic obstruction Mesoblastic nephroma Cardiac structural anomaly Tachyarrythmias Sacrococcygeal teratoma Hydrops |
|
Maternal–fetal infection Cytomegalovirus Toxoplasmosis Herpes simplex infection Parvovirus infection |
|
Aneuploidies, sex chromosome aneuploidies |
|
Maternal diabetes |
|
Placental tumours |
|
Bartter syndrome |
Table 2: Amniotic fluid analysis.
|
Tested infection |
Results |
Reference values (Garnier et. al 2010, Nature) |
Reference values (Bernie et al. Am J Obstet Gynecol 1974) |
|
Herpes simplex DNA PCR-RT |
negative |
||
|
Toxoplasma gondii DNA PCR-RT |
Negative |
||
|
Cytomegalovirus DNA PCR-RT |
Negative |
||
|
Ebstein Barr virus DNA PCR-RT |
negative |
||
|
Amniotic fluid biochemistry |
|
||
|
Protein |
1.96 g/l |
5.2 (2.3–9.9) g/l |
|
|
Potassium (K+) |
3.6 mmol/l |
3.8 (3.1–4.6) mmol/l |
4.0 ±0.2 mmol/l |
|
Sodium (Na+) |
144 mmol/l |
136 (129–141) mmol/l |
136.0 ± 6.8 mmol/l |
|
Chloride (Cl-) |
108 mmol/l |
111 (105–118) mol/l |
108.8 ±3.2 mmol/l |
|
Calcium (Ca2+) |
1.41 mmol/l |
1.47 (0.67–1.93) mmol/l |
7.4 ±2.2 (mg/dl) |
|
Karyotype |
46, XY |
|
|

References
- Hamza A, Herr D, Solomayer EF, Meyberg-Solomayer G (2013) Polyhydramnios: Causes, Diagnosis and Therapy. Geburtshilfe Frauenheilkd 73: 1241-1246. [Crossref]
- Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org, Dashe JS, Pressman EK, Hibbard JU (2018) SMFM Consult Series #46: Evaluation and management of polyhydramnios. Am J Obstet Gynecol 219: B2-B8. [Crossref]
- Bhat YR, Vinayaka G, Sreelakshmi K (2012) Antenatal bartter syndrome: a review. Int J Pediatr 2012: 857136. [Crossref]
- Fretzayas A, Gole E, Attilakos A, Daskalaki A, Nicolaidou P et al. (2013) Expanding the spectrum of genetic mutations in antenatal Bartter syndrome type II. Pediatr Int 55: 371-373. [Crossref]
- Koulouridis E, Koulouridis I (2015) Molecular pathophysiology of Bartter’s and Gitelman’s syndromes. World J Pediatr 11: 113-125. [Crossref]
- Kömhoff M, Laghmani K (2017) Pathophysiology of antenatal Bartter's syndrome. Curr Opin Nephrol Hypertens 26: 419-425. [Crossref]
- Gross I, Siedner-Weintraub Y, Simckes A, Gillis D (2015) Antenatal Bartter syndrome presenting as hyperparathyroidism with hypercalcemia and hypercalciuria: a case report and review. J Pediatr Endocrinol Metab 28: 943-946. [Crossref]
- Garnier A, Dreux S, Vargas-Poussou R, Oury JF, Benachi A et al. (2010) Bartter syndrome prenatal diagnosis based on amniotic fluid biochemical analysis. Pediatr Res 67: 300-303. [Crossref]
- Allaf B, Dreux S, Schmitz T, Czerkiewicz I, Le Vaillant C et al. (2015) Amniotic fluid biochemistry in isolated polyhydramnios: a series of 464 cases. Prenat Diagn 35: 1331-1335. [Crossref]
- Erfani H, Diaz-Rodriguez GE, Aalipour S, Nassr A, Rezaei A (2019) Amnioreduction in cases of polyhydramnios: Indications and outcomes in singleton pregnancies without fetal interventions. Eur J Obstet Gynecol Reprod Biol 241: 126-128. [Crossref]
- Wong AC, Chan LG (2014) Neonatal bartter syndrome. Med J Malaysia 69: 229-230. [Crossref]
- Tourne G, Collet F, Varlet MN, Billiemaz K, Prieur F et al. (2003) Prenatal Bartter's syndrome. Report of two cases. J Gynecol Obstet Biol Reprod (Paris) 32: 751-754. [Crossref]
- Massa G, Proesmans W, Devlieger H, Vandenberghe K, Van Assche A et al. (1987) Electrolyte composition of the amniotic fluid in Bartter syndrome. Eur J Obstet Gynecol Reprod Biol 24: 335-340. [Crossref]
- Harper LM, Cahill AG, Smith K, Macones GA, Odibo AO (2012) Effect of maternal obesity on the risk of fetal loss after amniocentesis and chorionic villus sampling. Obstet Gynecol 119: 745-751. [Crossref]
- Lewi L, Hanssens M, Spitz B, Deprest J (2004) Complete chorioamniotic membrane separation. Case report and review of the literature. Fetal Diagn Ther 19: 78-82. [Crossref]
- Puricelli E, Bettinelli A, Borsa N, Sironi F, Mattiello C et al. (2010) Long-term follow-up of patients with Bartter syndrome type I and II. Nephrol Dial Transplant 25: 2976-2981. [Crossref]
- Rudin A (1988) Bartter's syndrome. A review of 28 patients followed for 10 years. Acta Med Scand 224: 165-171. [Crossref]
