Chemokines are Underestimated in Preventing the Metastasizing and The Immune Elimination of Ovarian Cancer
A B S T R A C T
Nowadays the positive immune involvement in the eradication of tumor cells is assigned to the adaptive immune response. By awakening of in vivo responding T cells that are suppressed by the tumor and prevents immunological cure of the cancer. Normally activated T cells are well-ordered by several late occurring inhibitors to contain the response to the unknown invaders and spare the normal cells. The tumor strengthens this inhibitory response to escape from immune elimination. Immunotherapy is to unleash the full capacity of the adaptive immune system by blocking this inhibitor response by monoclonal antibodies but with the potential drawback of autoimmune phenomena. Cytokines and chemokines became in oblivion after their suspected necrosis of the tumor (TNF) did not fulfil their initial hope. Ovarian cancer is in most cases already metastasized to the peritoneum and omentum. Here, we show that on the one hand chemokines produced by Th2, CD8 and NK cells inhibit cancer spreading and thus leads to a better operability and better survival. Chemokine receptors are expressed by the tumor that are a decoy by binding chemokines that normally should attract antigen cross-presenting dendritic cells that start an enforced T cell response to replace the exhausted T cells.
Keywords
Ovarian cancer metastasis, chemokines, cytoreductive surgery, cytotoxic T cells, BDCA3 Dendritic cells
Introduction
Several papers describe that the presence of cytotoxic T cells (CTL) in ovarian cancer tissue will prolong their survival [1, 2]. Apparently, the tumor is an incentive for CD8 CTL’s to enter the microenvironment but only the Ag specific will enter the tumor. They need an integrin (CD103) expressed on the surface to enter the tumor bed [2, 3]. In ovarian cancer a few mutations are found. According to the tumor portal 94% of the ovarian tumors (http://www.tumorportal.org/tumor_types?ttype=OV) show TP53 mutations/deletions. A deletion of TP53 will not lead to an abundant immune recognition. So, there are only a low number of specific CD8 T-cells that have a tremendous effect on survival. The question arises how these few CTLs are able to impede the overwhelming number of tumor cells. Therefore, there should be another mechanism at work in concert with the CTLs that explains the clinical findings. The majority of the CTL are in the stromal compartment of the tumor. If those CTL that were alerted by the tumor but cannot penetrate the tumor tissue attribute to the production of chemokines remains elusive. But the overshoot of chemokines; CCL26; lymphotactins: XCL1 and XCL2; CCL2, CCL4, CCL5 that are found in the ascites and since the volume of ascites is several liters there should be large cell pool that produces them [4-7]. The tumor expresses several chemokine receptors among them CX3CR1 [8-10]. Here, we hypothesize that the tumor expresses chemokine receptors that by binding to chemokine CCL26 are unable to bind to membrane-bound fractalkine reducing their metastasis based on several reports [5, 11-13]. Less binding to the membrane-bound fractalkine abundantly expressed on peritoneum and omentum will result in less tumor spots and thus a better operability. Better operability causes a longer survival [14]. Tumor cells express also the lymphotactin receptor XCR1 [15, 16]. The chemokines XCL1 and XCL2 will bind to this decoy receptor. This will lower the quantity of the chemokines and result in less capacity to attract Ag cross-presenting dendritic cells (BDCA3) and will diminish new recruitment of T cells that should replace the inhibited T cells in the tumor [17].
Figure 1: (A) Overview by stitching several images to cover the whole tissue slide. Expression of cytokeratin 7 (green) by tumor cells and cytotoxic T cells (red) (white arrows: cytotoxic T cells in tumor). The nuclei are stained with DAPI (blue). (B) Close ups of a small part of the tumor showing the membranous staining of CD8 and the nuclei are stained by DAPI. The white arrow depicts an intratumoral CD8.
Materials and Methods
Ascites was obtained from stage III and IV high-grade serous ovarian cancer patients, one mucinous and one clear cell, before start of the treatment. The study was carried out in accordance with the guidelines and regulation of the Radboudumc and the WMO (Dutch law on Medical Research in Humans). Strictly according to their criteria, setting up and use of human tissue from a biobank does not fall under the WMO. In their point of view is stated that at the time of collecting human material, there is no specific question. This means that the research does not meet the definition of "medical research" according to the WMO. However, at the time of the release of the body material or data there is a specific research question. After all, the body material has already been collected with an earlier intervention. Some samples were obtained before written consent was needed. Ascites was considered as waste material and only oral informed consent was necessary. All patients gave this oral informed consent to help future patients and were aware that it was not for their own benefit but for research purposes. CCL26 was measured in ascites fluid using the CCL26 ELISA (R&D Systems, Inc. Minneapolis, USA). Immunohistochemistry was performed by a deparaffinized tissue section and after washing stained with monoclonal antibodies. Putative staining was observed with a Leica DMI6000 epi-fluorescence microscope and a metal halide EL6000 lamp. The pictures were taken using a DFC365FX CCD camera. Monoclonal antibody to CD8 (345774) was from BD Pharmingen, Vianen, The Netherlands.
Results and Discussion
Most patients with ovarian cancer that present themselves are diagnosed with advanced stage high-grade serous cancer and have a poor 5-year overall survival rate of just 30% [18]. Although in ovarian cancer tumor-infiltrating CD8+ T cells are found and linked with prolonged overall survival [1, 19]. But this influx of immune cells does not prevent a cure of the disease. High grade serous ovarian cancer remains the deadliest cancer in gynecological cancers. In most cases TP 53 is mutated in ± 96% of the cases while 9 other genes are mutated at a very low abundancy [20]. This leads to a limited number of targets for a cytotoxic immune response, which is reflected by the low response of immunotherapy [21]. In those cases where the immunotherapy was functional a durable response was measured [22]. Immune histochemistry of ovarian cancer show in a large overview of the tumor a few CD8 cells in the tumor and more in the stroma (Figure 1: A & B). In ascitic fluid from malignant ovarian cancer several chemo/cytokines were described soluble fractalkine; CCL26; lymphotactins: XCL1 and XCL2; CCL2, CCL4, CCL5 [4-7, 10, 12, 23]. Th2 cells generate IL-4-/IL-13 that stimulate vascular endothelial cells and these cells abundantly produce CCL26 [5, 24].
Table 1: Expression of CCL26/eotaxin3 in ascites
|
Patient |
CCL26 (pg/ml) |
tumor type |
stage |
Treatment* |
Result surgery |
|
1 |
3,8 |
serous |
3C |
NACT |
complete |
|
2 |
26,4 |
serous |
3C |
primary |
complete |
|
3 |
88,3 |
serous |
4 |
NACT |
incomplete |
|
4 |
4,2 |
serous |
3B |
primary |
optimal |
|
5 |
53,7 |
serous |
3C |
primary |
complete |
|
6 |
84,8 |
serous |
3C |
NACT |
complete |
|
7 |
218,1 |
serous |
3C |
primary |
complete |
|
8 |
9,7 |
serous |
3C |
NACT |
complete |
|
9 |
152,0 |
serous |
3C |
NACT |
complete |
|
10 |
0,0 |
serous |
3C |
NACT |
optimal |
|
11 |
0,0 |
serous |
4 |
NACT |
incomplete |
|
12 |
10,0 |
serous |
3C |
NACT |
complete |
|
13 |
0,0 |
serous |
3C |
primary |
optimal |
|
14 |
10,0 |
mucinous |
4 |
primary |
optimal |
|
15 |
4,9 |
serous |
4 |
NACT |
optimal |
|
16 |
61,5 |
clear cell |
3C |
NACT |
complete |
|
17 |
29,3 |
serous |
3C |
primary |
optimal |
*: treatment either primary cytoreduction followed by 6 cycles of advent chemotherapy (carboplatinum + paclitaxel), or neoadjuvant chemotherapy (NACT), followed by interval debulking (cytoreductive surgery), followed by 3 cycles of djuvant chemotherapy
** result surgery: complete: no residual disease; optimal: residual tumor < 1cm; incomplete: residual tumor > 1cm
However, if CCL26 was present in ascites is unknown. Upon analysis, in several cases CCL26 is found in ascites (Table 1). Three patients did not express CCL26 and did not have a complete removal of the tumor. Patients with stage 4 did not have a complete removal. The other patients CCL26 blocked the fractalkine receptor and should lead to less seeding of the tumor cell on the peritoneum because the receptor is occupied by CCL26 (Figure 2). CCL26 appears an important chemokine in spreading of the tumor in the abdomen. If CCL26 is found in 1 to 10-liter ascites it indicates that CCL26 is produced in large quantities. Moreover, the production is enough to saturate the receptor and the remainder will appear in the ascites. The fractalkine receptor CX3CR1 is expressed on tumor cells allowing the circulating tumor cells to bind to cell-bound fractalkine that is present on mesothelial cells of the peritoneum and omentum. Indeed, spreading of tumor nests is different for individual patients as reported during surgical removal of the tumor. Patients with a better operability survive longer [14, 25]. CCL26 produced by NK and CD8 cells can bind to the CX3CR1 receptor [5].
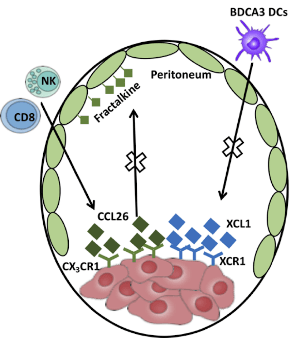
Figure 2: A diagram of the expression of chemokines and their receptors and the mechanism of less seeding of tumor cells to the peritoneum or omentum. Binding of XCL1 and XCL2 to the tumor expressed XCR1 lowering their quantity.
Moreover, activated T cells produce IL-4 and IL-13 that stimulate vascular endothelial cells to produce CCL26 [24]. So, the presence of CCL26 should diminish nesting of circulating tumor cells and those patients should have a better operability. Since CCL26 is produced by NK and CD8 cells it could explain the better survival when CD8 cells are present in the tumor. Thus, CCL26 reduces the docking places for tumor cells on the peritoneum and omentum. Support for such a mechanism comes from a synthetic inhibitor for CX3CR1 (JMS-17-2) that destroys metastatic formation of spots of breast cancer cells [26]. When tumors show XCR1 expression and secreted XCL1 or XCL2 by NK cells, will be bound by the tumor causing lowering the level of the chemokines in the fluid phase and leading to less influx of BDCA3 dendritic cells in the tumor microenvironment. Initially we found a very low BDCA3 positive cells from ascites, which made us search for the cause of this low influx. BDCA3 DCs express the chemokine receptor XCR1, which binds the XCL1/2 chemokines and penetrate the tumor and start an immune response. The putative XCR1 expression on tumors could lower the level of XCL1/2 in ascites. This explains the differences of BDCA3 dendritic cells found in the cell fraction of ascites fluid. Immunology comprises several cells and protein molecules to eliminate invading pathogens and inhibit the response after invading pathogen is eradicated.
Nowadays much effort in cancer treatment is the use of inhibition by monoclonal antibodies to membrane proteins that normally keep the immune system in check. Although much success is gathered it uses only one entity of the immune system. To expand the armament to Cancer also the other entities of the immune system should be exploited. Here, we highlighted the chemokines as influencers of cancer growth. The ensemble of the clinical data, cyto/chemokine levels, B- and T-cells, and the myeloid branch of the immune system, the important targets for each patient should be revealed leading to a better individualized treatment and prolonged survival. We propose to treat patients directly after their diagnosis with an antagonist to CX3CR1 to prevent any further adherence of circulating tumor cells. BDCA3 DCs should be collected by apheresis and loaded with cancer stem cell antigens and infused in the patient [27]. In this way, the tumor battled T cells are refreshed by fully functional T cells.
Conclusion
In patients where Cancer that is infiltrated with CD8 cytotoxic T cells have a prolonged survival. It is surprising that a few CD8 cells compared to the overwhelming number of cancer cells managed this prolonged survival. After a rigorous search in the literature a few papers that showed that ovarian cancer expressed CX3CR1 that bound CCL26 (eotaxin-3) to hamper the adhesion to the peritoneum. CCL26 is produced by activated CD8, NK cells and activated Th2 cells. Normally CX3CR1 will bind to membrane bound fractalkine expressed on peritoneum and omentum, the favorite metastasis site. Due to CCL26 that blocks binding to fractalkine less cancer spots and a more optimal cytoreductive surgery leads to a better overall survival. Tumor cells express XCR1 that binds to the lymphotactins XCL1 and XCL2 and serves as a decoy receptor lowering their concentration. Those chemokines normally attract BDCA3 dendritic cells. Less BDCA3 DC will enter the tumor area and less T cells will be activated that should replace the tumor inhibited T cells.
Author Contributions
P.Z. collected the specimen and assigned the diagnosis and the gradation; RT designed the study and wrote the paper. Both were involved in obtaining the data.
Funding
This research received no external funding.
Conflicts of interest
The authors declare no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 02, Dec 2019Accepted: Wed 18, Dec 2019
Published: Mon 30, Dec 2019
Copyright
© 2023 Ruurd Torensma. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2019.04.04
Author Info
Petra L.M.Zusterzeel Ruurd Torensma
Corresponding Author
Ruurd TorensmaDepartment of Tumorimmunology, Radboud Molecular Life Sciences, Radboudumc, Nijmegen, Netherlands
Figures & Tables
Table 1: Expression of CCL26/eotaxin3 in ascites
|
Patient |
CCL26 (pg/ml) |
tumor type |
stage |
Treatment* |
Result surgery |
|
1 |
3,8 |
serous |
3C |
NACT |
complete |
|
2 |
26,4 |
serous |
3C |
primary |
complete |
|
3 |
88,3 |
serous |
4 |
NACT |
incomplete |
|
4 |
4,2 |
serous |
3B |
primary |
optimal |
|
5 |
53,7 |
serous |
3C |
primary |
complete |
|
6 |
84,8 |
serous |
3C |
NACT |
complete |
|
7 |
218,1 |
serous |
3C |
primary |
complete |
|
8 |
9,7 |
serous |
3C |
NACT |
complete |
|
9 |
152,0 |
serous |
3C |
NACT |
complete |
|
10 |
0,0 |
serous |
3C |
NACT |
optimal |
|
11 |
0,0 |
serous |
4 |
NACT |
incomplete |
|
12 |
10,0 |
serous |
3C |
NACT |
complete |
|
13 |
0,0 |
serous |
3C |
primary |
optimal |
|
14 |
10,0 |
mucinous |
4 |
primary |
optimal |
|
15 |
4,9 |
serous |
4 |
NACT |
optimal |
|
16 |
61,5 |
clear cell |
3C |
NACT |
complete |
|
17 |
29,3 |
serous |
3C |
primary |
optimal |
*: treatment either primary cytoreduction followed by 6 cycles of advent chemotherapy (carboplatinum + paclitaxel), or neoadjuvant chemotherapy (NACT), followed by interval debulking (cytoreductive surgery), followed by 3 cycles of djuvant chemotherapy
** result surgery: complete: no residual disease; optimal: residual tumor < 1cm; incomplete: residual tumor > 1cm


References
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H et al. (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 102: 18538-18543. [Crossref]
- Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH (2014) Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 20: 434-444. [Crossref]
- Flies DB, Higuchi T, Harris JC, Jha V, Gimotty PA et al. (2016) Immune checkpoint blockade reveals the stimulatory capacity of tumor-associated CD103(+) dendritic cells in late-stage ovarian cancer. Oncoimmunology 5: e1185583. [Crossref]
- Muralidhar GG, Barbolina MV (2013) Chemokine receptors in epithelial ovarian cancer. Int J Mol Sci 15: 361-376. [Crossref]
- Nakayama T, Watanabe Y, Oiso N, Higuchi T, Shigeta A et al. (2010) Eotaxin-3/CC chemokine ligand 26 is a functional ligand for CX3CR1. J Immunol 185: 6472-6479. [Crossref]
- Kroczek RA, Henn V (2012) The role of XCR1 and its ligand XCL1 in antigen cross-presentation by murine and human dendritic cells. Front Immunol 3: 14. [Crossref]
- Siros E, Duttagupta P, Dangaj D, Li H, Frank R et al. (2015) The Ovarian Cancer Chemokine Landscape Is Conducive to Homing of Vaccine-Primed and CD3/CD28-Costimulated T Cells Prepared for Adoptive Therapy. Clin Cancer Res 21: 2840-2850. [Crossref]
- Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL (2005) The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res 65: 10355-10362. [Crossref]
- Benhadjeba S, Edjekouane L, Sauve K, Carmona E, Tremblay A (2018) Feedback control of the CXCR7/CXCL11 chemokine axis by estrogen receptor alpha in ovarian cancer. Mol Oncol 12: 1689-1705. [Crossref]
- Kim M, Rooper L, Xie J, Kajdacsy-Balla AA, Barbolina MV (2012) Fractalkine receptor CX (3) CR1 is expressed in epithelial ovarian carcinoma cells and required for motility and adhesion to peritoneal mesothelial cells. Mol Cancer Res 10: 11-24. [Crossref]
- Gurler Main H, Xie J, Muralidhar GG, Elfituri O, Xu H et al. (2017) Emergent role of the fractalkine axis in dissemination of peritoneal metastasis from epithelial ovarian carcinoma. Oncogene 36: 3025-3036. [Crossref]
- Xie J, Gurler H, Burdette J, Barbolina M (2013) CX3CR1, a fractalkine receptor, can regulate peritoneal dissemination of epithelial ovarian cancer cells. Cancer Res 73: B72.
- El-Shazly AE, Doloriert HC, Bisig B, Lefebvre PP, Delvenne P et al. (2013) Novel cooperation between CX3CL1 and CCL26 inducing NK cell chemotaxis via CX3CR1: a possible mechanism for NK cell infiltration of the allergic nasal tissue. Clin Exp Allergy 43: 322-331. [Crossref]
- Torres D, Wang C, Kumar A, Bakkum-Gamez JN, Weaver AL et al. (2018) Factors that influence survival in high-grade serous ovarian cancer: A complex relationship between molecular subtype, disease dissemination, and operability. Gynecol Oncol 150: 227-232. [Crossref]
- Khurram SA, Whawell SA, Bingle L, Murdoch C, McCabe BM et al. (2010) Functional expression of the chemokine receptor XCR1 on oral epithelial cells. J Pathol 221: 153-163. [Crossref]
- Kim M, Rooper L, Xie J, Rayahin J, Burdette JE (2012) The lymphotactin receptor is expressed in epithelial ovarian carcinoma and contributes to cell migration and proliferation. Mol Cancer Res 10: 1419-1429. [Crossref]
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M et al. (2010) Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 207: 1273-1281. [Crossref]
- Wright JD, Chen L, Tergas AI, Patankar S, Burke WM et al. (2015) Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol 125: 1345-1352. [Crossref]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M et al. (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348: 203-213. [Crossref]
- Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474: 609-615. [Crossref]
- Bronte G, Cicero G, Sortino G, Pernice G, Catarella MT et al. (2014) Immunotherapy for recurrent ovarian cancer: a further piece of the puzzle or a striking strategy? Expert Opin Biol Ther 14: 103-114. [Crossref]
- Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D et al. (2019) Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol Oncol 152: 243-250. [Crossref]
- Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC Jr et al. (2015) Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 15: 668-679. [Crossref]
- Blanchard C, Durual S, Estienne M, Emami S, Vasseur S et al. (2005) Eotaxin-3/CCL26 gene expression in intestinal epithelial cells is up-regulated by interleukin-4 and interleukin-13 via the signal transducer and activator of transcription 6. Int J Biochem Cell Biol 37: 2559-2573. [Crossref]
- Griffiths CT, Fuller AF (1978) Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am 58: 131-142. [Crossref]
- Shen F, Zhang Y, Jernigan DL, Feng X, Yan J (2016) Novel Small-Molecule CX3CR1 Antagonist Impairs Metastatic Seeding and Colonization of Breast Cancer Cells. Mol Cancer Res 14: 518-527. [Crossref]
- Wefers C, Schreibelt G, Massuger L, de Vries IJM, Torensma R (2018) Immune Curbing of Cancer Stem Cells by CTLs Directed to NANOG. Front Immunol 9: 1412. [Crossref]
