Census of Cardiac Uptake on Routine Bone Scintigraphy, a Methodology to Assess the Prevalence of the TTR Amyloidosis
A B S T R A C T
Objective: Transthyretin cardiac amyloidosis is a rapidly progressive disease, remains underdiagnosed, and has long been considered a rare disease. Diagnosis decision making cannot be based on echography and MRI findings alone because they lack accuracy. However, it requires invasive test of myocardial or fat pad biopsies. Thus, the diagnosis is challenging, and the non-invasive scintigraphic technique using bone-seeking radiopharmaceuticals has become a cornerstone in the workup revealing a myocardial uptake. Our work consists of a single institution retrospective study aiming to estimate the bone scintigraphies, and the prevalence of the transthyretin cardiac amyloidosis in our population.
Methods: We have carried out a census of the incidental cardiac uptake on routine bone scintigraphy in 2211 consecutive patients enrolled between 2009 and 2020, mostly performed for oncologic purposes. The visual analysis of the scintigraphic images classifies the cardiac uptake into 3 levels of gradation and evokes the diagnosis of the TTR cardiac amyloidosis in grades 2 and 3.
Results: Different grades of myocardial uptake were observed in 1.72 % of all patients. Prevalence of uptake was 0.37% in the 50th, increased dramatically at the 60th (2.49%), and reached 4% above the age of 80. The diagnostic grades 2 and 3 cardiac uptakes were identified in 5 patients (0.23% of overall patients) ranging between 56- and 91-y-old. Our results concord with the trend of disease increases with age.
Conclusion: The careful analysis of the bone scintigraphy of all incoming adult patients should allow to make the diagnosis of the TTR cardiac amyloidosis at a preclinical stage and establish new effective treatments. Our results provide an estimate of the number of patients that could be diagnosed and improve the awareness of the corresponding prevalence in our population. In this way, multicentric studies should be implemented by enrolling large cohorts of bone scintigraphy cases.
Keywords
Technetium 99m, transthyretin cardiac amyloidosis, bone-seeking radiopharmaceuticals, scintigraphy
Introduction
Cardiac amyloidosis (CA) occurs when amyloid molecules replace normal cardiac myocytes [1]. It is one of the leading causes of restrictive heart disease and can affect the electrical conduction system of the heart [2, 3]. It is clinically divided into an amyloid light chain (AL), transthyretin mutant (TTRm), and Transthyretin wild types (TTRwt) [4]. AL amyloid is the most common form, related to a plasma cell clone producing light chains that will deposit in various organs [5]. The TTR CA, is caused by the dissociation of the transthyretin tetramer into monomers [4-6]. Untreated CA is a rapidly progressive disorder with median survival from diagnosis of less than 6 months for the AL form and 3 to 5 years for the TTR form [7-9]. Patients with TTR CA, the focus of our study, most often have multiple medical visits as well as multiple hospital admissions and long stays but remain underdiagnosed in most cases [10]. The association of heart failure with other non-cardiac features such as bilateral carpal tunnel syndrome, peripheral neuropathy, and tendon rupture should raise the suspicion of CA [11-13]. It leads in many cases, to postural hypotension and electrocardiogram (ECG) changes, mainly low voltage [14, 15]. Echocardiography (ECHO) shows bilateral atrial dilatation, left ventricular hypertrophy, and diastolic dysfunction [16-18]. There is a reduction of the global longitudinal strain, and bull’s eye mapping demonstrates an apical sparing, which does not exist in patients with hypertension or hypertrophic cardiomyopathy [18].
Magnetic resonance imaging (MRI) is useful for visualizing high T1 signals and signs suggestive of diffuse fibrosis commonly seen in amyloidosis [19]. Diagnosis of CA cannot be based on ECHO and MRI findings alone because they lack accuracy, and invasive tests provide the confirmation of diagnosis by performing myocardial or fat pad biopsies [20]. Troponin and proBNP play a role in the prognosis of AL and TTR CA and can suggest the development of heart failure [21]. Serum-free light chains and urine immunofixation should be performed to rule out the AL type of amyloidosis in all suspected patients [22]. Thus, the diagnosis is challenging, and scintigraphy visualizing a myocardial uptake of bone-seeking radiopharmaceuticals (BSR) has become a cornerstone in the diagnosis of TTR CA, providing high sensitivity and specificity [23]. Cardiac biopsies, which were the gold standard tests for diagnosis, are now only used for extremely rare cases [24].
The cardiac uptake of BSR can be observed in a variety of conditions and is not only limited to TTR CA. Patients with prostate cancer, end-stage renal disease on hemodialysis, myocarditis, and restrictive cardiomyopathies have an increased probability of heart uptake as compared to individuals with normal conditions [25-28]. The hydroxychloroquine long-term treatment in rheumatology could be associated with an increase in heart uptake [29, 30]. The presence of myocardial tracer uptake on bone scintigraphy should suggest several differential diagnoses next to the TTR CA [28].
CA is considered a rare disease, with a prevalence of 5.5 per 10000 persons in the United States and 1 per 6000 persons in Sweden [4, 31]. In a large retrospective study made on a national scale in Japan between 2010 and 2018, the prevalence of CA subtypes of TTRwt, TTRm, and AL was 191.1, 5.1, and 21.2 per million respectively [32]. Considering only those patients who were also diagnosed with heart failure, CA turns out to be more frequent in men in all sub-types, being 55.2%, 59%, and 59.4%, respectively [5, 6]. The low prevalence of CA begins to be unmasked, concerning the TTRw type, notably, by the recent adoption of non-invasive diagnostic modalities of radionuclide imaging [23].
Our work consists of a single institution (Mount Lebanon teaching hospital) retrospective study aiming to estimate, in the Lebanese population, the prevalence of the TTR CA. It is based on the incidental cases revealed on routine bone scintigraphy, with the major clinical indication being the diagnosis of bone metastasis. This helps to reduce the risk of underdiagnosis or misdiagnosis of this entity because newer treatments and targeted therapies are being developed and have high hopes of stopping the disease progression.
Methods
This study enrolls the results of the retrospective interpretation of bone scintigraphy examinations, using 8MBq/Kg of Technetium 99m labeling hydroxyl methyl diphosphonate (99mTc-HMDP), performed in our department between 2009 and 2020. The study has conducted according to the principles outlined in the Declaration of Helsinki. We used the Interpretation criteria based on the visual evaluation of the myocardial uptake of radiopharmaceuticals by comparison to costal bone uptake according to the Perugini scale, which is divided into 4 grades: in grade 0 myocardial uptake is absent, in grade 1 myocardial uptake is lower than bone one, in grade 2 myocardial and bone uptakes are equivalent, and in grade 3 myocardial uptake is higher than bone one [33, 34]. Grades 2 and 3 are diagnostic of TTR CA, while grade 1 corresponds to possible CA if additional features are met. Bone scintigraphy images were interpreted by an experienced nuclear medicine specialist blinded to patient information.
The medical records of the positive patients were reviewed to exclude pathologies other than TTR CA, which could be a cause of myocardial fixation. True positive cases underwent echocardiography to demonstrate restrictive cardiomyopathy. The Microsoft Office Excel 2007 software was used in the statistical analysis. The continuous variables are expressed as mean ± SD. The categorical variables are expressed as percentages.
Results
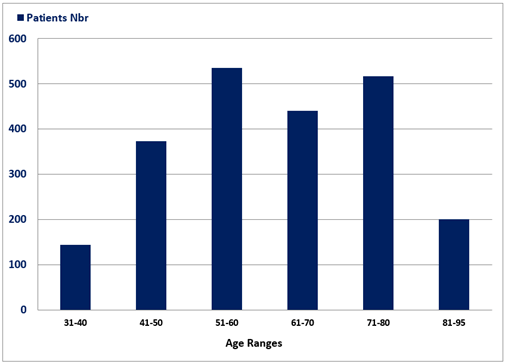
A total of 2211 consecutive cases of bone scintigraphy realized in patients ranging between 31- and 95-y-old (mean age = 61.40 ± 15.42) were included in this study, 1261 are females (57%) and 950 are males (43%). Figure 2 histogram represents the age range distribution of patients, with the majority being aged between 41 and 80-y-old. The indication to perform bone scintigraphy was bone metastasis workup in the majority of patients (81.58%), while bone pain and rheumatologic diseases were evaluated in the remaining minority of patients (18.42%).
Table 1: Illustrates the demographic data of positive cases
with cardiac uptake, arranged gender (females first) in ascending grade.
|
CASES |
AGE (Years) |
SEX |
INDICATION |
GRADE |
|
1 |
38 |
female |
Breast cancer |
1 |
|
2 |
49 |
female |
Bone pain |
1 |
|
3 |
58 |
female |
Breast cancer |
1 |
|
4 |
62 |
female |
Breast cancer |
1 |
|
5 |
66 |
female |
Unknown primary cancer |
1 |
|
6 |
67 |
female |
Bone pain |
1 |
|
7 |
67 |
female |
Breast cancer |
1 |
|
8 |
70 |
female |
Prosthesis |
1 |
|
9 |
76 |
female |
Breast cancer |
1 |
|
10 |
76 |
female |
Breast cancer |
1 |
|
11 |
78 |
female |
Colon cancer |
1 |
|
12 |
79 |
female |
Breast cancer |
1 |
|
13 |
79 |
female |
Breast cancer |
1 |
|
14 |
80 |
female |
Lung cancer |
1 |
|
15 |
82 |
female |
Breast cancer |
1 |
|
16 |
82 |
female |
Breast cancer |
1 |
|
17 |
41 |
male |
Bone pain |
1 |
|
18 |
61 |
male |
Prostate cancer |
1 |
|
19 |
62 |
male |
Prostate cancer |
1 |
|
20 |
67 |
male |
Prostate cancer |
1 |
|
21 |
68 |
male |
Prostate cancer |
1 |
|
22 |
72 |
male |
Prostate cancer |
1 |
|
23 |
74 |
male |
Prostate cancer |
1 |
|
24 |
74 |
male |
Prostate cancer |
1 |
|
25 |
75 |
male |
Lung cancer |
1 |
|
26 |
76 |
male |
Prostate cancer |
1 |
|
27 |
77 |
male |
Bone pain |
1 |
|
28 |
79 |
male |
Bladder cancer |
1 |
|
29 |
80 |
male |
Prostate cancer |
1 |
|
30 |
83 |
male |
Prostate cancer |
1 |
|
31 |
84 |
male |
Bone pain |
1 |
|
32 |
84 |
male |
Prostate cancer |
1 |
|
33 |
85 |
male |
Prostate cancer |
1 |
|
34 |
40 |
female |
Breast cancer |
2 (FP*, cardiac infarct) |
|
35 |
56 |
female |
Breast cancer |
2 |
|
36 |
61 |
female |
Breast cancer |
2 |
|
37 |
66 |
male |
Prostate cancer |
2 |
|
38 |
74 |
male |
Prostate cancer |
2 (FP*, blood pool activity) |
|
39 |
84 |
male |
Bone pain |
3 |
|
40 |
91 |
male |
Prostate cancer |
3 |
*FP: false positive.
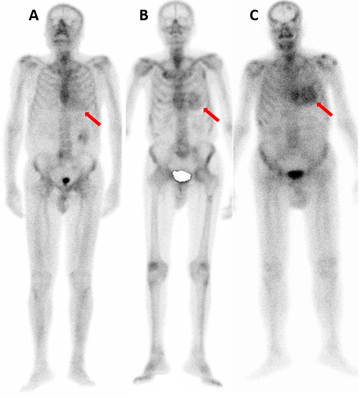
Figure 1: Represents images of whole skeleton 99mTc-HMDP scintigraphy performed in three different patients, in whom A) grade 1, B) grade 2, and C) grade 3 myocardial uptakes are identified; the red arrow in each image indicates the myocardial activity.
Figure 2: Histogram representing the age ranges distribution of the 2211 patients, with the majority being aged between 41 and 80-y-old.
Table 1 illustrates the demographic characteristics of patients with myocardial uptake, which is observed in 40 patients (1.8% of the total population), 19 females (48% of patients with uptake), and 21 males (52% of patients with uptake). Cardiac uptake is found in 2.11% of males and 1.43% of females in the total population. Different grades of cardiac uptake were detected (Tables 1 & 2): grade 1 in 33 patients (Figure 1A; 86.4% of the positive cases and 1.49% of the total population); grade 2 in 5 patients, while 3 are interpreted as true positive (TP) for TTR CA (Figure 1B; 7.89% of the positive cases and 0.14% of the total population) and 2 as false positive (FP) being cardiac infarct (40 y old female) and blood pool activity (74 y old male with renal failure); grade 3 in 2 patients (Figure 1C; 5.26% of the positive cases and 0.09% of the total population). Patients with grade 0 uptake had a mean age lower than patients with positive uptake of all grades, 61.24 y ± 15.36 and 69.89 y ± 12.75, respectively. The mean age of patients with grade 3 is much higher than grade 2, being 87.5-y and 61-y old, respectively. The ages of grade 1 patients are dispersed in a wide range (38 y-91 y), as compared to the narrow age ranges of grade 2 (56-66 y) and grade 3 patients (84 y-91 y) (Tables 2 & 3).
Table 2: Illustrates the number, percentage, age range, and
mean age of negative cases as well as of positive cases according to the grade
of cardiac uptake.
|
Grade |
Patients
Number |
Population
% |
Cases
% |
Age
Range |
Mean
Age |
|
|
Negative Cases |
0 |
2171 |
98.19 |
100 |
30
- 91 |
61.24 |
|
Positive Cases
|
1 |
33 |
1.49 |
86.4 |
38
- 85 |
71.24 |
|
2 |
3 |
0.14 |
7.89 |
56
- 66 |
61.00 |
|
|
3 |
2 |
0.09 |
5.26 |
84
- 91 |
87.50 |
|
|
All positive |
38 |
1.72 |
100 |
38
- 91 |
69.89 |
Table 3: Illustrates according to the age ranges, the number
and percentage of patients with all positive grades, low grade 1, and high
grades 2 and 3.
|
AGE Ranges |
Patients No. |
All Positive Grades No. and % |
Grade 1 No. and % |
Grade 2 and 3 No. and % |
|
31-40 |
144 |
2 and 1.39% |
1 and 0.69% |
0 and 0% (1 FP*) |
|
41-50 |
373 |
2 and 0.54% |
2 and 0.54% |
0 and 0% |
|
51-60 |
536 |
2 and 0.37% |
1 and 0.19% |
1 and 0.19% |
|
61-70 |
441 |
11 and 2.49% |
9 and 2.04% |
2 and 0.45% |
|
71-80 |
517 |
15 and 2.9% |
14 and 2.71% |
0 and 0% (1 FP*) |
|
81-91 |
200 |
8 and 4% |
6 and 3% |
2 and 1% |
*FP:
false positive.
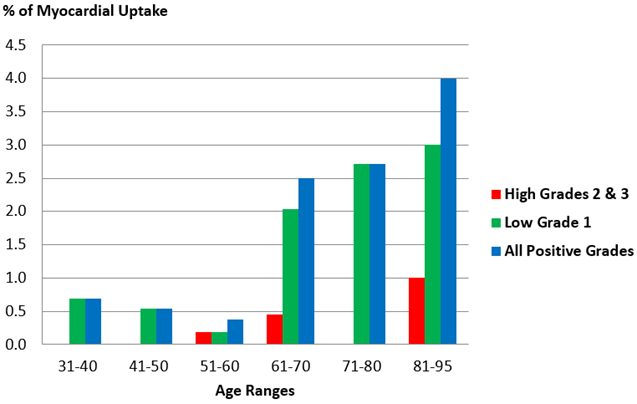
Figure 3 histogram illustrates the percentage of cumulative cases of all grades (blue column), separated low grade 1 cases (green column), and separated high grades 2 and 3 cases (red column), according to the different age ranges. It demonstrates that the incidence of all grades of cardiac uptake increases with age, being minimal in 50th (0.37%), dramatically increases in 60th (2.49%), to become approximately 10 folds superior above 81 y (4%). In a paradoxical and most likely hazardous sense, the percentage in the 30th age range is mildly higher than in the 40th and 50th. In all ranges, the histogram pattern depends essentially on the number of grade 1 patients, in whom the pathological significance of the cardiac uptake is difficult to be evaluated without further clinical, biological, and radiological investigations. Incidence of high grades 2 and 3 that are diagnostic for TTR CA commences in the 50th age range and increases with age.
Figure 3: Histogram illustrating the percentage of cumulated all positive grade cases (blue column), separated low grade 1 cases (green column), and separated high grades 2 and 3 cases (red column), according to the different age ranges; the profile of positive cases depends essentially on the percentage of grade 1 patients; incidence of high grades 2 and 3 that are diagnostic for TTR CA commences in 50th age range and increases with age.
Discussion
The establishment of a correct diagnosis of TTR CA requires multiple exploratory modalities. ECG can help by detecting pseudo infarct patterns and discordant left ventricular hypertrophy [15]. ECHO classically shows signs of left ventricular hypertrophy and apical sparing detected on the global longitudinal strain and valvular anomalies with thickening of the valves and interatrial septum [4, 17, 18]. MRI also has a role in completing the diagnostic study of a patient with suspected CA; it is, however, a sophisticated imaging technique, not usually practiced in many hospitals, and of long duration, as machine occupation time, limiting the number of studies that can be performed per day [19]. Likewise, increasing the number of MRI sequences further prolongs the examination time, which hinders accessibility. Another drawback is that MRI does not provide instant information as with the trans-thoracic echography and requires post-processing by an experienced radiologist to complete the entire test. The gold standard diagnostic test is still the myocardial biopsy, which is an extremely invasive procedure, but the diagnostic methodology of TTR CA is changing in the last decade in favour of the 99mTc-PYP scintigraphy [24, 35].
In TTR CA, the sensitivity and specificity of scintigraphy using BSR, as compared to ECHO and MRI, are much higher, evaluated at 92.2% and 95.4%, respectively [22, 36]. Multiple radio-tracers are used to diagnose TTR CA, such as 99mTc-DPD, 99mTc-PYP, and 99mTc-HMDP, which have high affinity to TTR amyloid fibrils in the cardiac myocytes and rarely to AL amyloidal fibrils and can also differentiate CA from other infiltrative diseases such as sarcoidosis [37]. The hypothesis of binding of BSR to the myocardium was linked to the presence of calcium in amyloidal fibrils [38]. In doubt amyloidosis cases, additional laboratory tests are performed to eliminate the possibility of AL amyloid subtype [25]. This relatively new radionuclide methodology is revolutionary and will have positive effects on diagnosis, prognosis, and early treatment leading to a reduction in mortality [39]. The 99mTc labeling PYP or HMDP is readily available in any nuclear medicine facility, with simple radiopharmacy preparation. The scintigraphic imaging protocol is straightforward, including planar anterior, left anterior oblique, and left lateral views, performed 1 hour and 3 hours after intravenous injection of the tracer [37]. Single-photon emission computed tomography (SPECT) is made 1 hour after injection [37]. SPECT reconstruction is important to confirm the localization of the radiotracer in the myocardium [40].
In some patients (renal failure), significant blood pool activity can be noted in cardiac cavities, and imaging is repeated at a later time point to allow adequate blood pool clearance [40]. The key step is to distinguish between the blood pool in the left ventricle and true myocardial uptake [41]. A quantitative heart-to-contralateral lung ratio has been proposed for 99mTc-pyrophosphate, with values greater than 1.5 being highly specific for TTR [41]. In the absence of histology, some studies have provided proof of very high reliability of the scintigraphy to diagnose TTR CA, with a specificity of 99% when: 1) ECHO and MRI show signs of heart failure, left ventricular hypertrophy, or atrial dilatation; 2) myocardial uptake of BSR is classified in 2 or 3 grade; 3) AL amyloidosis is excluded by laboratory tests consisting of a serum-free light chain and urine immunofixation [11, 16, 17, 22, 33, 34, 42]. Tissue sampling is required in highly suspicious TTR CA if ECG, ECHO, MRI, and SPECT are negative [24].
18F-NaF PET tracer of skeleton targets microcalcifications in amyloidal fibers and is able to differentiate TTR from AL cardiac amyloidosis [43]. However, this radionuclide imaging of the skeleton is not used in the routine workup of bone metastases and cannot serve in screening studies. 18F-florbetapir and 18F-florbetaben are PET tracers considered for evolving nuclear imaging techniques that have been used to study brain amyloidosis in the case of Alzheimer's disease [44]. Though, 18F-florbetapir has a non-specific affinity for normal myocardium and reveals no difference in the intensity of uptake in at-risk patients for TTR CA and controls [45]. Moreover, these PET radiotracers cannot differentiate AL from TTR CA [46]. The 123I-SAP SPECT tracer, a circulating serum amyloid P component, is capable of binding to amyloid fibers of all types but is unable to be conclusive because of the high background of blood pooling [42]. Even though CA is considered a senile systemic disease of aged individuals with more severity at the ages of 80 and above, several publications reported cases, in young and middle-aged adults, at the 30th, 40th, 50th, and 60th of age [47-50]. The youngest published case is a 34-y-old male from India with TTR CA and a 38-y-old female from China with AL CA [48-50]. This fact has stimulated us to investigate TTR CA not only in the elderly but in young adults starting from the 30th.
Most of our patients with positive cardiac uptake are non-specific grade 1, as shown in (Table 2) and histogram of (Figure 3) (33 patients of 38, 86.84%), in whom TTR CA could not be confirmed or excluded by the clinical exam, the ECG, or radiologic imaging of the heart. Only 5 among 38 positive patients are high grade 2 or 3 cardiac uptake (7.89% and 5.26% respectively), a diagnostic finding of TTR CA supported by the clinical and ECHO examinations of the heart.
In our cohort, the mean age of patients with cardiac uptake (69.5-y-old) is higher than the mean age of all populations (61.4-y-old). The incidence of myocardial uptake increases with age, being 1.72% in all our cohorts. It starts from 0.69% in the 30th age range, dramatically increases to 2.04% in the 60th, and reaches 4% above the age of 80. Our data confirms once more the trend of increasing TTR CA with age. High grades 2 and 3 cardiac uptakes associated with higher age (Figure 3) is demonstrated by a Spanish study enrolling 1114 patients aged above 75 y, with a percentage being more significant corresponding to 2.7% of cases [51]. The limited number of 5 cases of high-grade uptake disclosed in our cohort of 2211 patients (0.23%) ranging between 56- and 91-y-old (0.29% of patients in this age range with a mean age of 71.6-y-old) is explained by the low mean age of our population. A recently published Italian study enrolling 4228 cases of bone scintigraphy, predominantly indicated for oncologic purposes, shows high grades of cardiac uptake in 0.54% of patients with a mean age of 83 ± 5 y, a percentage closer to our result than that of Spanish one [52].
TTR cardiac amyloidosis is well known to affect the older population, and it was proven by examining myocardial cells in deceased patients from other conditions [53, 54]. The cases of amyloidosis remain underdiagnosed due to minor awareness of this complex tissue disorder that overlaps with other conditions such as heart failure and ischaemic heart diseases [55]. It is estimated that patients above 60 y experiencing left ventricular hypertrophy and heart failure with preserved ejection fraction, have underlying TTR CA in 13% to 18% of cases [56]. Coincidently, our results reveal a dramatic increase in cardiac uptake of 99mTc-HMDP (Figure 3) from this age of 60-y-old, which could be a threshold drawing attention to a non-negligible probability of having early TTR CA and justifying close clinical follow-up. Moreover, the mean age of patients with grade 2 cardiac uptake (61-y-old) is lower than grade 3 (87.5-y-old), with possible collinearity between the intensity of the tracer uptake and the progression of the disease over time. The youngest case presenting diagnostic cardiac uptake corresponds to a 56-y-old female with grade 2, a fact indicating that the disease occurs in middle-aged adults. Subsequently, we recommend screening the TTR CA on routine bone scintigraphy well before the ages of 70 or 75-y-old as generally adopted by other studies [51, 52].
In our study, the incidence of myocardial uptake was 2.11% in males and 1.43% in females. Moreover, cases of cardiac uptake are more males than females (52% and 48% respectively), despite enrolling more cases of female patients. This finding is in concordance with the literature data indicating a mildly higher prevalence of TTR CA in older males than in females [7, 52]. This study has limitations from being monocentric, enrolling cases of cancer patients mainly, and identifying a small number of high-grade diagnostic cardiac uptake. The reliable assessment of the prevalence of TTR CA in our population, and the extrapolation of our recommendation to communities other than ours should engage in multicentric clinical trials that involve large cohorts of patients, not specifically focused on cancer patients.
Conclusion
Cardiac amyloidosis is a rare disease but most often remains underdiagnosed. The scintigraphic technique using BSR, allows visualization of myocardial uptake, makes the diagnosis easier, avoids invasive approach, and goes beyond sophisticated medical imaging techniques, such the MRI.
Our work shows that the incidence of myocardial uptake increases with age in the same manner as the TTR CA. To diagnose the TTR CA at a preclinical stage, the results of our study, combined with those of literature data, teach us to analyse the myocardial uptake of bone scintigraphy of all incoming patients minutely, starting from 30-y-old. Scintigraphic Screening for TTR CA in young patients is most likely not cost-effective due to the low likelihood of having specific myocardial uptake. It would be better to reserve this technique for middle-aged and older adults, suspected of having cardiac amyloidosis for diagnosis making and differentiating between TTR and AL CA. The setting of non-specific grade 1 cardiac uptake, whose frequency is high from the age of 60-y-old, requires continuous follow-up by a research team, and repeated scintigraphy in order to determine the fraction of patients with early TTR CA.
Data of our work provide an estimate of the number of patients that could be diagnosed with TTR CA, assessed in a mono-centric database of the department of nuclear medicine of Mount Lebanon Hospital over a period of 11 y. Improving awareness about the prevalence of this disease should improve diagnosis and treatment. In this way, multicentric studies should be implemented by enrolling large cohorts of bone scintigraphy cases.
Conflicts of Interest
None.
Funding
None.
Acknowledgments
Not Applicable.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 31, May 2022Accepted: Wed 15, Jun 2022
Published: Thu 30, Jun 2022
Copyright
© 2023 Feras Chehade. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2022.02.01
Author Info
Antoine El Khoury Samer Nasr Feras Chehade
Corresponding Author
Feras ChehadeDepartment of Nuclear Medicine, Faculty of Medical Sciences, Lebanese University, Hadath, Baabda, Lebanon
Figures & Tables
Table 1: Illustrates the demographic data of positive cases
with cardiac uptake, arranged gender (females first) in ascending grade.
|
CASES |
AGE (Years) |
SEX |
INDICATION |
GRADE |
|
1 |
38 |
female |
Breast cancer |
1 |
|
2 |
49 |
female |
Bone pain |
1 |
|
3 |
58 |
female |
Breast cancer |
1 |
|
4 |
62 |
female |
Breast cancer |
1 |
|
5 |
66 |
female |
Unknown primary cancer |
1 |
|
6 |
67 |
female |
Bone pain |
1 |
|
7 |
67 |
female |
Breast cancer |
1 |
|
8 |
70 |
female |
Prosthesis |
1 |
|
9 |
76 |
female |
Breast cancer |
1 |
|
10 |
76 |
female |
Breast cancer |
1 |
|
11 |
78 |
female |
Colon cancer |
1 |
|
12 |
79 |
female |
Breast cancer |
1 |
|
13 |
79 |
female |
Breast cancer |
1 |
|
14 |
80 |
female |
Lung cancer |
1 |
|
15 |
82 |
female |
Breast cancer |
1 |
|
16 |
82 |
female |
Breast cancer |
1 |
|
17 |
41 |
male |
Bone pain |
1 |
|
18 |
61 |
male |
Prostate cancer |
1 |
|
19 |
62 |
male |
Prostate cancer |
1 |
|
20 |
67 |
male |
Prostate cancer |
1 |
|
21 |
68 |
male |
Prostate cancer |
1 |
|
22 |
72 |
male |
Prostate cancer |
1 |
|
23 |
74 |
male |
Prostate cancer |
1 |
|
24 |
74 |
male |
Prostate cancer |
1 |
|
25 |
75 |
male |
Lung cancer |
1 |
|
26 |
76 |
male |
Prostate cancer |
1 |
|
27 |
77 |
male |
Bone pain |
1 |
|
28 |
79 |
male |
Bladder cancer |
1 |
|
29 |
80 |
male |
Prostate cancer |
1 |
|
30 |
83 |
male |
Prostate cancer |
1 |
|
31 |
84 |
male |
Bone pain |
1 |
|
32 |
84 |
male |
Prostate cancer |
1 |
|
33 |
85 |
male |
Prostate cancer |
1 |
|
34 |
40 |
female |
Breast cancer |
2 (FP*, cardiac infarct) |
|
35 |
56 |
female |
Breast cancer |
2 |
|
36 |
61 |
female |
Breast cancer |
2 |
|
37 |
66 |
male |
Prostate cancer |
2 |
|
38 |
74 |
male |
Prostate cancer |
2 (FP*, blood pool activity) |
|
39 |
84 |
male |
Bone pain |
3 |
|
40 |
91 |
male |
Prostate cancer |
3 |
*FP: false positive.
Table 2: Illustrates the number, percentage, age range, and
mean age of negative cases as well as of positive cases according to the grade
of cardiac uptake.
|
Grade |
Patients
Number |
Population
% |
Cases
% |
Age
Range |
Mean
Age |
|
|
Negative Cases |
0 |
2171 |
98.19 |
100 |
30
- 91 |
61.24 |
|
Positive Cases
|
1 |
33 |
1.49 |
86.4 |
38
- 85 |
71.24 |
|
2 |
3 |
0.14 |
7.89 |
56
- 66 |
61.00 |
|
|
3 |
2 |
0.09 |
5.26 |
84
- 91 |
87.50 |
|
|
All positive |
38 |
1.72 |
100 |
38
- 91 |
69.89 |
Table 3: Illustrates according to the age ranges, the number
and percentage of patients with all positive grades, low grade 1, and high
grades 2 and 3.
|
AGE Ranges |
Patients No. |
All Positive Grades No. and % |
Grade 1 No. and % |
Grade 2 and 3 No. and % |
|
31-40 |
144 |
2 and 1.39% |
1 and 0.69% |
0 and 0% (1 FP*) |
|
41-50 |
373 |
2 and 0.54% |
2 and 0.54% |
0 and 0% |
|
51-60 |
536 |
2 and 0.37% |
1 and 0.19% |
1 and 0.19% |
|
61-70 |
441 |
11 and 2.49% |
9 and 2.04% |
2 and 0.45% |
|
71-80 |
517 |
15 and 2.9% |
14 and 2.71% |
0 and 0% (1 FP*) |
|
81-91 |
200 |
8 and 4% |
6 and 3% |
2 and 1% |
*FP:
false positive.
References
1. Martinez-Naharro A,
Hawkins PN, Fontana M (2018) Cardiac amyloidosis. Clin Med (Lond) 18:
s30-s35. [Crossref]
2. Hassan W,
Al-Sergani H, Mourad W, Tabbaa R (2005) Amyloid heart disease. New frontiers
and insights in pathophysiology, diagnosis, and management. Tex Heart Inst
J 32: 178-184. [Crossref]
3. Strouse C,
Briasoulis A, Fonseca R, Jethava Y (2019) Approach to a patient with cardiac
amyloidosis. J Geriatr Cardiol 16: 567-574. [Crossref]
4. Gilstrap LG,
Dominici F, Wang Y, El-Sady MS, Singh A et al. (2019) Epidemiology of Cardiac
Amyloidosis-Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare
Beneficiaries in the United States. Circ Heart Fail 12: e005407. [Crossref]
5. Müller AMS, Geibel
A, Neumann HPH, Kühnemund A, Schmitt‐Gräff
A et al. (2006) Primary (AL) amyloidosis in plasma cell disorders. Oncologist
11: 824-830. [Crossref]
6. Escher F, Senoner
M, Doerler J, Zaruba MM, Messner M et al. (2020) When and how do patients with
cardiac amyloidosis die?. Clin Res Cardiol 109: 78-88. [Crossref]
7. Ruberg FL, Berk JL
(2012) Transthyretin (TTR) cardiac amyloidosis. Circulation 126:
1286-1300. [Crossref]
8. Yamada T, Takashio
S, Arima Y, Nishi M, Morioka M et al. (2020) Clinical characteristics and
natural history of wild-type transthyretin amyloid cardiomyopathy in Japan. ESC
Heart Fail 7: 2829-2837. [Crossref]
9. Merlini G,
Palladini G (2013) Light chain amyloidosis: the heart of the problem. Haematologica
98: 1492-1495. [Crossref]
10. Mesquita ET, Jorge
AJL, Souza Junior CV, de Andrade TR (2017) Cardiac Amyloidosis and its New
Clinical Phenotype: Heart Failure with Preserved Ejection Fraction. Arq Bras
Cardiol 109: 71-80. [Crossref]
11. Milandri A, Farioli
A, Gagliardi C, Longhi S, Salvi F et al. (2020) Carpal tunnel syndrome in
cardiac amyloidosis: implications for early diagnosis and prognostic role
across the spectrum of aetiologies. Eur J Heart Fail 22: 507-515. [Crossref]
12. Shin SC,
Robinson-Papp J (2012) Amyloid neuropathies. Mt Sinai J Med 79: 733-748.
[Crossref]
13. Geller HI, Singh A,
Alexander KM, Mirto TM, Falk RH (2017) Association Between Ruptured Distal
Biceps Tendon and Wild-Type Transthyretin Cardiac Amyloidosis. JAMA 318:
962-963. [Crossref]
14. Palma JA,
Gonzalez-Duarte A, Kaufmann H (2019) Orthostatic hypotension in hereditary
transthyretin amyloidosis: epidemiology, diagnosis and management. Clin
Auton Res 29: 33-44. [Crossref]
15. Garrahy I, Forman
D, Swierczynski S (2019) Low voltage criteria EKG as a harbinger of systemic
disease. J Community Hosp Intern Med Perspect 9: 226-229. [Crossref]
16. Fitzgerald BT,
Scalia GM, Cain PA, Garcia MJ, Thomas JD (2011) Left atrial size--another
differentiator for cardiac amyloidosis. Heart Lung Circ 20: 574-578. [Crossref]
17. Cariou E, Smires
YB, Victor G, Robin G, Ribes D et al. (2017) Diagnostic score for the detection
of cardiac amyloidosis in patients with left ventricular hypertrophy and impact
on prognosis. Amyloid 24: 101-109. [Crossref]
18. Salman K, Cain PA,
Fitzgerald BT, Sundqvist MG, Ugander M (2017) Cardiac Amyloidosis Shows
Decreased Diastolic Function as Assessed by Echocardiographic Parameterized
Diastolic Filling. Ultrasound Med Biol 43: 1331-1338. [Crossref]
19. Banypersad SM (2019)
The Evolving Role of Cardiovascular Magnetic Resonance Imaging in the
Evaluation of Systemic Amyloidosis. Magn Reson Insights 12:
1178623X19843519. [Crossref]
20. Quarta CC,
Gonzalez-Lopez E, Gilbertson JA, Botcher N, Rowczenio D et al. (2017)
Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur
Heart J 38: 1905-1908. [Crossref]
21. Grogan M,
Dispenzieri A, Gertz MA (2017) Light-chain cardiac amyloidosis: strategies to
promote early diagnosis and cardiac response. Heart 103: 1065-1072. [Crossref]
22. Milani P, Merlini
G, Palladini G (2018) Light Chain Amyloidosis. Mediterr J Hematol Infect Dis
10: e2018022. [Crossref]
23. Kuria IM, Gitau SN,
Makhdomi KB (2019) Bone scintigraphy imaging of cardiac amyloidosis. World J
Nucl Med 18: 314-316. [Crossref]
24. Prochorec-Sobieszek
M, Bilińska ZT, Grzybowski J, Michalak E, Jakubowska E et al. (2005) Cardiac
amyloidosis diagnosed by endomyocardial biopsy. Clinical, histopathological, immunohistochemical
and ultrastructural studies. Kardiol Pol 63: 20-35. [Crossref]
25. Chadrawar S, George
M, Al-Akraa M, Herber M, Buscombe J (2009) Myocardial uptake of Tc-99m HDP in a
patient with prostate carcinoma. Nucl Med Rev Cent East Eur 12: 78-80. [Crossref]
26. Cesani F,
Villanueva-Meyer J (1996) Intense myocardial and lung uptake of
99m-Tc-pyrophosphate using single photon emission computed tomography in a
patient with end-stage renal disease and secondary hyperparathyroidism. Int
Urol Nephrol 28: 569-574. [Crossref]
27. Matsumori A, Kadota
K, Kawai C (1980) Technetium-99m pyrophosphate uptake in experimental viral
perimyocarditis. Sequential study of myocardial uptake and pathologic
correlates. Circulation 61: 802-807. [Crossref]
28. Travascio L,
Ciancamerla M, Colandrea M, Di Nicola AD, Giacomobono S et al. (2008) A finding
of myocardial uptake at a bone scintigraphy with Tc-99m HDP. Clin Ter
159: 419-420. [Crossref]
29. Dorbala S, Cuddy S,
Falk RH (2020) How to Image Cardiac Amyloidosis: A Practical Approach. JACC
Cardiovasc Imaging 13: 1368-1383. [Crossref]
30. Wakfie-Corieh CG,
López NR, Sanz Saiz-Pardo M, Castejón MJP, Vilacosta I (2021) Not All Heart
Uptakes on 99mTc-DPD Scintigraphy Are Amyloidosis: Chloroquine-Induced
Cardiomyopathy. Clin Nucl Med 46: e188-e189. [Crossref]
31. Lindmark K, Pilebro
B, Sundström T, Lindqvist P (2021) Prevalence of wild type transtyrethin cardiac
amyloidosis in a heart failure clinic. ESC Heart Fail 8: 745-749. [Crossref]
32. Winburn I, Ishii T,
Sumikawa T, Togo K, Yasunaga H (2019) Estimating the Prevalence of
Transthyretin Amyloid Cardiomyopathy in a Large In-Hospital Database in Japan. Cardiol
Ther 8: 297-316. [Crossref]
33. Wollenweber T,
Rettl R, Kretschmer-Chott E, Rasul S, Kulterer O et al. (2020) In Vivo
Quantification of Myocardial Amyloid Deposits in Patients with Suspected
Transthyretin-Related Amyloidosis (ATTR). J Clin Med 9: 3446. [Crossref]
34. Perugini E,
Guidalotti PL, Salvi F, Cooke RMT, Pettinato C et al. (2005) Noninvasive
etiologic diagnosis of cardiac amyloidosis using
99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll
Cardiol 46: 1076-1084. [Crossref]
35. Poterucha TJ, Elias
P, Bokhari S, Einstein AJ, DeLuca A et al. (2021) Diagnosing Transthyretin
Cardiac Amyloidosis by Technetium Tc 99m Pyrophosphate: A Test in Evolution. JACC
Cardiovasc Imaging 14: 1221-1231. [Crossref]
36. Treglia G,
Glaudemans AWJM, Bertagna F, Hazenberg BPC, Erba PA et al. (2018) Diagnostic
accuracy of bone scintigraphy in the assessment of cardiac
transthyretin-related amyloidosis: a bivariate meta-analysis. Eur J Nucl Med
Mol Imaging 45: 1945-1955. [Crossref]
37. Singh V, Falk R, Di
Carli MF, Kijewski M, Rapezzi C et al. (2019) State-of-the-art radionuclide
imaging in cardiac transthyretin amyloidosis. J Nucl Cardiol 26:
158-173. [Crossref]
38. Fathala A (2020)
Incidentally detected cardiac amyloidosis on 99mTc-MDP bone scintigraphy. Radiol
Case Rep 15: 705-708. [Crossref]
39. Park J, Egolum U,
Parker S, Andrews E, Ombengi D et al. (2020) Tafamidis: A First-in-Class
Transthyretin Stabilizer for Transthyretin Amyloid Cardiomyopathy. Ann
Pharmacother 54: 470-477. [Crossref]
40. Ren C, Ren J, Tian
Z, Du Y, Hao Z et al. (2021) Assessment of cardiac amyloidosis with
99mTc-pyrophosphate (PYP) quantitative SPECT. EJNMMI Phys 8: 3. [Crossref]
41. Meristoudis G,
Ilias I, Keramida G (2020) Potential diagnostic pitfalls of bone scintigraphy
in transthyretin-related amyloidosis. World J Nucl Med 19: 313-314. [Crossref]
42. Bokhari S, Shahzad
R, Castaño A, Maurer MS (2014) Nuclear imaging modalities for cardiac
amyloidosis. J Nucl Cardiol 21: 175-184. [Crossref]
43. Trivieri MG, Dweck
MR, Abgral R, Robson PM, Karakatsanis NA et al. (2016) 18F-Sodium
Fluoride PET/MR for the Assessment of Cardiac Amyloidosis. J Am Coll Cardiol
68: 2712-2714. [Crossref]
44. Yeo JM, Waddell B,
Khan Z, Pal S (2015) A systematic review and meta-analysis of (18)F-labeled
amyloid imaging in Alzheimer's disease. Alzheimers Dement (Amst) 1:
5-13. [Crossref]
45. Sperry BW, Bock A,
DiFilippo FP, Donnelly JP, Hanna M et al. (2021) Pilot Study of F18-Florbetapir
in the Early Evaluation of Cardiac Amyloidosis. Front Cardiovasc Med 8:
693194. [Crossref]
46. Wagner T, Page J,
Burniston M, Skillen A, Ross JC et al. (2018) Extracardiac 18F-florbetapir
imaging in patients with systemic amyloidosis: more than hearts and minds. Eur
J Nucl Med Mol Imaging 45: 1129-1138. [Crossref]
47. Abeykoon JP, Paludo
J, Dispenzieri A, Gertz MA, Dingli D et al. (2017) Outcome of very young (≤40
years) patients with immunoglobulin light chain (AL) amyloidosis. Amyloid
24: 50-51. [Crossref]
48. Ghosh S, Khanra D,
Krishna V, Thakur AK (2021) Wild type transthyretin cardiac amyloidosis in a
young individual: A case report. Medicine (Baltimore) 100: e25462. [Crossref]
49. Gao M, Liu Q, Chen
L (2019) Cardiac amyloidosis as a rare cause of heart failure: A case report. Medicine
(Baltimore) 98: e15036. [Crossref]
50. Zhang Q, Qiao Y,
Yan D, Deng Y, Zhang M et al. (2020) Myocardial amyloidosis following multiple
myeloma in a 38-year-old female patient: A case report. Open Med (Wars)
15: 396-402. [Crossref]
51. Mohamed-Salem L,
Santos-Mateo JJ, Sanchez-Serna J, Hernández-Vicente Á, Reyes-Marle R et al.
(2018) Prevalence of wild type ATTR assessed as myocardial uptake in bone scan
in the elderly population. Int J Cardiol 270: 192-196. [Crossref]
52. Bianco M, Parente
A, Biolè C, Righetti C, Spirito A et al. (2021) The prevalence of TTR cardiac
amyloidosis among patients undergoing bone scintigraphy. J Nucl Cardiol
28: 825-830. [Crossref]
53. Brunjes DL, Castano
A, Clemons A, Rubin J, Maurer MS (2016) Transthyretin Cardiac Amyloidosis in
Older Americans. J Card Fail 22: 996-1003. [Crossref]
54. Palladini G,
Merlini G (2013) Systemic amyloidoses: what an internist should know. Eur J
Intern Med 24: 729-739. [Crossref]
55. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C (2017) Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation 135: 1357-1377. [Crossref]
56. Devesa A, Blasco AC, Lázaro AMP, Askari E, Lapeña G et al. (2021) Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy. ESC Heart Fail 8: 2856-2865. [Crossref]
