Carvacrol: A Brief Analysis of Molecular Properties and Their Therapeutic Potential
A B S T R A C T
This mini review presents some research involving carvacrol, a phenolic monoterpenoid that has been investigated due to its low toxicity and its applicability in the synthesis of derivatives with important biological properties. The molecular properties of carvacrol were calculated to broaden the discussion about its bio dynamicity.
Keywords
Carvacrol, molecular properties, anti-inflammatory activity, central nervous system
Pharmacokinetic Profile and Molecular Properties of Carvacrol
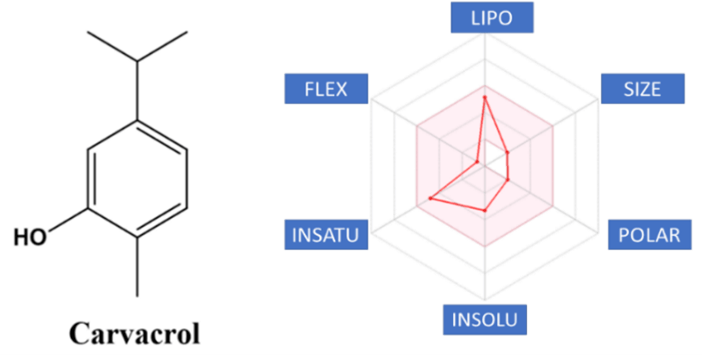
Carvacrol (2-methyl-5- (1-methyl ethyl) is a phenolic monoterpene found as a constituent of the essential oil of several families, such as: Lamiaceae, Euforbiaceae and Verbanaceae [1]. In the species Origanum minutiflorum and Origanum onites the carvacrol composes 92% of the essential oil [2]. The literature has shown that carvacrol presents great bio dynamicity, with emphasis on antimicrobial, anti-inflammatory, antitumor and anti-hepatotoxic activity [3, 4]. This apparent therapeutic potential of carvacrol can be explained due to its druglike properties, with emphasis on its physical and chemical properties and pharmacokinetic profile. The main physicochemical properties of a small molecule capable of altering its pharmacotherapeutic profile are the partition coefficient, which expresses the relationship between its hydro profile and liposolubility, and the ionization coefficient, expressed by pKa, degree of relative contribution of the neutral and ionized species [5].
Considering that the great majority of orally active drugs are passively absorbed, having to transpose the lipid bilayer that constitutes the hydrophobic environment of the biological membranes, the importance of the physicochemical properties lipophilicity and pKa, so that the drug plasma concentrations capable of replicating the biological effect evidenced in in vitro experiments [6]. In contrast, the process of oral absorption of a drug is dependent on its concentration in solution, a phenomenon that is favored by its relative water solubility profile. This dichotomy requires that a drug, or new drug-candidate prototype, should exhibit balanced physicochemical properties in order to adjust to the characteristics of each of the phases covered in the biophase [7]. Carvacrol has interesting physicochemical properties, as can be seen in (Table 1). The rationalization of the results can be done using the parameters proposed by Lipinski, Ghose, Veber, Egan and Muegge.
The Lipinski rule or rule of five (RO5) proposes a small absorption or permeation when the molecule has more than 5 hydrogen bonding donors, more than 10 hydrogen bonding acceptors, molecular weight greater than 500 Daltons and calculated log P (Clog P) greater than 5, all parameters being multiples of five [8]. Lipinski et al. demonstrated that orally administered drugs are far more likely to reside in areas of chemical space defined by a limited range of molecular properties [9]. In addition to Lipinski's rules, Veber et al. (2002) concluded that the number of rotatable bonds should be ≤ 10 and the polar surface area ≤ 140 Å2 [10]. Additionally, Egan et al. (2002) included the prerequisites TPSA> 131.6Å or log P> 5.88 [11]. As can be seen in (Table 1), carvacrol does not violate any of the rules of Lipinski, Veber and Egan, suggesting a possible therapeutic potential, to be better investigated using other experimental and theoretical models.
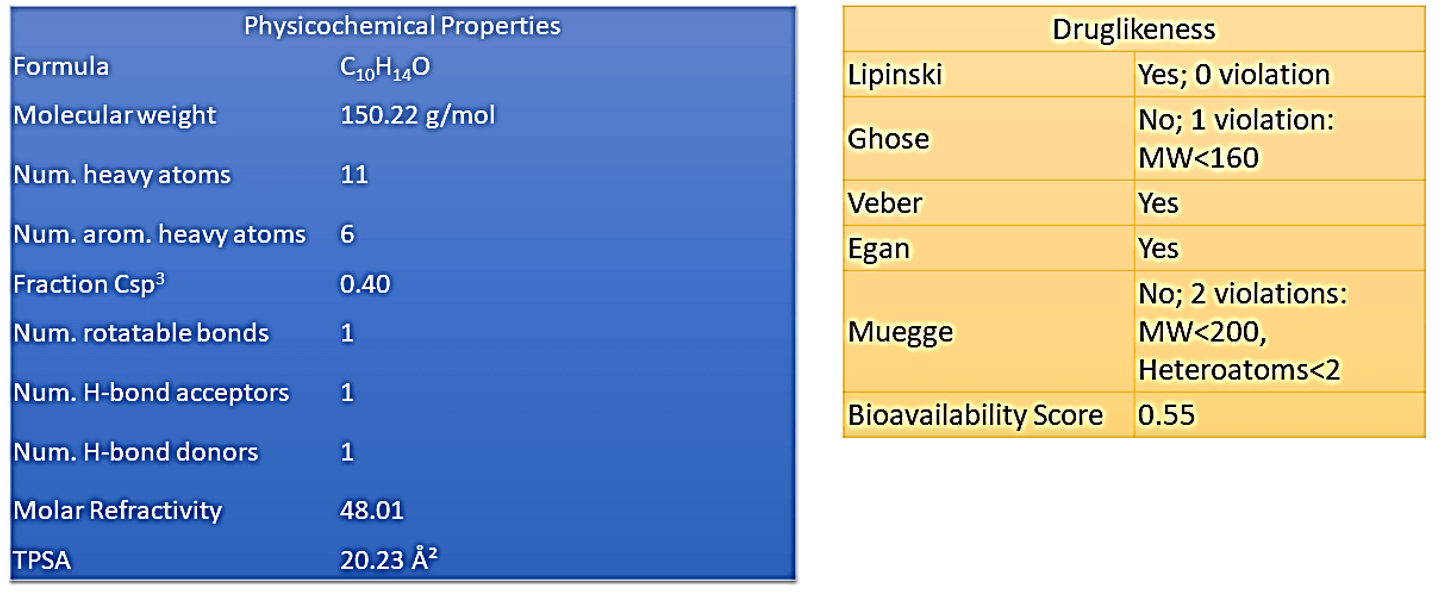
Once administered orally, the rate and extent of drug absorption along the gastrointestinal tract are highly dependent on physicochemical factors (solubility, polymorphism, pKa, lipophilicity), physiological (gastrointestinal pH, gastrointestinal transit time), and of the formulation (particle size, excipients, pharmaceutical form), directly influencing the systemic bioavailability [12]. Solubility is a property that directly influences the release and absorption of drugs, thus playing a significant role in bioavailability. For a drug to reach a specific therapeutic target, the molecules must be soluble in the physiological gut fluid. With the dissolved material, it is possible that absorption occurs in specific regions along the gastrointestinal tract [13]. As desired, carvacrol has an interesting set of pharmacokinetic properties, involving gastrointestinal (GI) absorption, ability to permeate the blood-brain barrier (BBB permeant), and a reasonable inhibition profile of CYP450 complex enzymes (Figure 1).
Figure 1: General scheme of pharmacokinetic and pharmacodynamic properties.
Most of the drugs have a lipophilic character and, at physiological pH, remain non-ionized or partially ionized. Due to these characteristics, they would tend to remain in the body, since they would be reabsorbed in the kidneys, after glomerular filtration [14]. In order to eliminate these exogenous substances, the organism can use enzymatic systems normally used for the degradation of endogenous substances. Thus, biotransformation is the enzymatic transformation of drugs into metabolites with more hydrophilic characteristics, aiming to facilitate excretion by the organism [15].
The biotransformation of drugs can be divided into two phases. Phase I consists of the reactions of oxidation, reduction and hydrolysis, always causing a structural modification of the drug, which in most cases can lead to its inactivation [16]. In the case of prodrug administration, phase I will be instrumental in generating the pharmacologically active substance. In phase II, known as the conjugation phase, reactions of conjugation of the drug with endogenous substances occur, aiming at facilitating its excretion. The phases I and II processes are independent, i.e. the drug can only undergo phase I or phase II reactions, or both, sequentially [17]. Generally, the phase I reactions introduce a relatively reactive group, such as the hydroxyl group, into the molecule, and this functional group will then serve as the point of attack for the conjugating system, which attaches to it a larger substituent, such as a glucuronyl group, sulfate or acetyl [18].
The organ where most biotransformation reactions occur is the liver, as it has several enzymes or specialized enzyme complexes. Among them, we highlight the monooxygenases of the CYP enzyme complex [19]. CYP is primarily responsible for the biotransformation of drugs in the human organism and is present mainly in the smooth plasma reticulum (microsomal fraction) of hepatocytes and can also be found in other organs such as lungs and kidneys. CYP is a protein with a heme prosthetic group (or ferro-porphyrin group) and belongs to the group of monooxygenases, which are enzymes that catalyze reactions in which an oxygen atom of the O2 molecule is incorporated into the molecule [20]. Monooxygenases require two substrates that act as reducers of the two oxygen atoms of O2. The main substrate (in this case the drug) receives one of two oxygen atoms and the co-substrate (in the case of CYP is nicotinamide adenine dinucleotide phosphate - NADPH) provides hydrogen atoms to reduce the second oxygen atom to water [21].
The oxidative system Cytochrome P450 (CYP450) comprises 57 genes that encode enzymes, the most important being CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A5, which are responsible for metabolizing more than 90% of drugs [22]. The cytochrome p450 superfamily is responsible for the metabolism of various medications and as the present polymorphism may increase or decrease this metabolism [23]. In the process of evaluating a drug candidate, Bioavailability Radar (Figure 2) is a highly relevant tool that makes use of six physicochemical properties: lipophilicity, size, polarity, solubility, flexibility and saturation [24]. The pink area represents the optimal range for each properties (lipophilicity: XLOGP3 between −0.7 and +5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å2, solubility: log S not higher than 6, saturation: fraction of carbons in the sp3 hybridization not less than 0.25, and flexibility: no more than 9 rotatable bonds. To improve the bioavailability of carvacrol, size, polarity and flexibility parameters can be improved by organic semi-synthesis.
Figure 2: Bioavailability radar from carvacrol.
Vasorelaxant Effect of Carvacrol
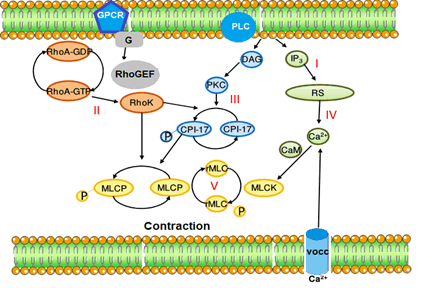
Thymol and carvacrol similarly inhibited contraction induced by CaCl2 in aortic rings depolarized by K+, suggesting that these terpenoids may interfere with the contraction produced by influx of Ca2+ through Ca2+ -dependent channels. There are reports that thymol and carvacrol suppressed currents of L-type Ca2+ in canine and human ventricular cardiomyocytes (Magyar et al., 2004), however it is unlikely that suppression of this Ca2+ influx may be involved in the vasorelaxant effects described in the present work, although this action cannot be completely ruled out. It should be noted that thymol and carvacrol reduced, with similar potencies, contractions induced in aortic rings under Ca2+ free conditions as much as they inhibited electromechanical and pharmaco-mechanical couplings. Thus, together, these results allow us to suggest a common site of action for thymol and carvacrol in both couplings, as a direct inhibitory effect on contractile proteins (Figure 3).
Figure 3: Mechanism of the vasorelaxant effect of carvacrol.
Anti-Inflammatory Activity
Lima et al., showed the anti-inflammatory action of carvacrol in a model of inflammation induced in mice by reducing the expression of IL-1band PGE2, important mediators of the inflammatory process, with a consequent increase in cytokine expression IL-10 [3]. In addition, CVC can inhibit cyclooxygenase, a limiting factor for prostaglandin synthesis, which justifies its anti-inflammatory and analgesic activity [4].
Guimarães et al., when evaluating the mechanism of anti-inflammation of carvacrol in the body of mice with carrageenan-induced hypernociception, attributed its anti-inflammatory activity to inhibition of the formation of the inflammatory mediator inflammatory cytokine TNF-α and the modulation of central nitric oxide NO pathways, with no effect by prostaglandin E2 (PGE2) and dopamine [25]. In studies carried out by Liang & Lu it was also demonstrated that the main mechanism of anti-inflammatory action of thymol is related to its ability to inhibit the production of the inflammatory cytokines TNF-α, IL-6 and IL-1β, which are associated with the cholinergic pathway, since the release of acetylcholine favors the inhibition of inflammatory cytokines, in addition to the modulation performed in the central nitric oxide NO pathways and by eliminating the expression of cyclooxygenase -2 (COX-2) [26, 27]. Hotta et al., also proved that carvacrol has anti-inflammatory activity associated with suppression of the COX-2 enzyme and activation of peroxisome proliferating receptors (PPARs) α and γ [28].
Central Nervous System Activity
In recent years, the significant anxiolytic and antidepressant activity of carvacrol has been reported. The main mode of action of this compound is being related to an action on GABAergic neurons through the GABAA receptor, similarly to benzodiazepines that have high affinity for these receptors. Melo et al. (2010) observed through tests performed on mice that oral administration of carvacrol favors the reduction of anxiety, since similarly to diazepam there was a significant increase in all parameters analysed by the elevated cross test (number of entries and total entries in open and closed arms) and without promoting effects on locomotor activity. The results demonstrated that the anxiolytic effect of the compound is related to its action on the GABAA receptor in a similar way to benzodiazepines. Other studies have suggested that the pharmacological action of carvacrol is related to the ability to modulate GABAergic inotropic receptors together with chlorine channels. Since the connection of this monoterpene to the GABA in the nervous system significantly increased chloride absorption (Tong & Coats, 2010). The evaluation of the effects antidepressants from the administration of carvacrol in mice, demonstrated that the compound has antidepressant activity associated with the dopaminergic system, probably by stimulating the D1 and D2 receptors. (Melo et al., 2011).
In addition, carvacrol was able to decrease excitability in the peripheral nervous system by blocking the compound action potential (PAC). Gonçalves et al. (2010) related this inhibitory effect to the ability to block voltage-gated sodium channels (NaV) in the sciatic nerve. The authors observed that this blocking ability varies in relation to the structure of the compounds, since carvacrol has a greater inhibitory effect in relation to limonene that does not have oxygen or hydroxyl. The ability of this compound to change its characteristics through the modification of its ligands increases its applicability, since by changing the substitutes of its chemical structures, it is possible to direct the drugs directly to the target without affecting other organisms.
Article Info
Article Type
Review ArticlePublication history
Received: Wed 18, Nov 2020Accepted: Tue 01, Dec 2020
Published: Wed 16, Dec 2020
Copyright
© 2023 Aldo Sena de Oliveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJMC.2020.01.10
Author Info
Corresponding Author
Aldo Sena de OliveiraDepartment of Exact Sciences and Education, Federal University of Santa Catarina- UFSC, Blumenau, Santa Catarina, Brazil
Figures & Tables

References
- Verma RS, Padalia RC, Chauhan A, Verma RK, Yadav AK et al. (2010) Chemical diversity in Indian oregano (Origanum vulgare L.). Chem Biodivers 7: 2054-2064. [Crossref]
- Aydin Y, Kutlay O, Ari S, Duman S, Uzuner K et al. (2007) Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med 73: 1365-1371. [Crossref]
- Lima MDS, Quintans Júnior LJ, de Santana WA, Martins Kaneto C, Pereira Soares MB et al. (2013) Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol 699: 112-117. [Crossref]
- Suntres ZE, Coccimiglio J, Alipour M (2015) The Bioactivity and Toxicological Actions of Carvacrol. Crit Rev Food Sci Nutr 55: 304-318. [Crossref]
- de Waterbeemd HV (2008) Physicochemical Properties in Drug Profiling. 25-52.
- Peetla C, Stine A, Labhasetwar V (2009) Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol Pharm 6: 1264-1276. [Crossref]
- Savjani KT, Gajjar AK, Savjani JK (2012) Drug solubility: importance and enhancement techniques. ISRN Pharm 2012: 195727. [Crossref]
- Santos VLDA, Gonsalves A, Araújo CRM (2018) Abordagem Didática Para O Desenvolvimento de Moléculas Bioativas: Regra Dos Cinco de Lipinski e Preparação de Heterociclo 1,3,4-Oxadiazol Em Forno de Micro-Ondas Doméstico. Quim Nova.
- Barret R (2018) Lipinski’s Rule of Five. Therapeut Chemist.
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW et al. (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 45: 2615-2623. [Crossref]
- Egan WJ, Merz KM Jr, Baldwin JJ (2000) Prediction of drug absorption using multivariate statistics. J Med Chem 43: 3867-3877. [Crossref]
- Li S, He H, Parthiban LJ, Yin H, Serajuddin ATM (2005) IV-IVC Considerations in the Development of Immediate-Release Oral Dosage Form. J Pharm Sci 94: 1396-1417. [Crossref]
- Stegemann S, Leveiller F, Franchi D, de Jong H, Lindén H (2007) When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci 31: 249-261. [Crossref]
- Shugarts S, Benet LZ (2009) The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res 26: 2039-2054 [Crossref]
- Kebamo S, Tesema S (2016) The Role of Biotransformation in Drug Discovery and Development. J Drug Metab Toxicol.
- Testa B, Pedretti A, Vistoli G (2012) Reactions and enzymes in the metabolism of drugs and other xenobiotics. Drug Discov Today 17: 549-560. [Crossref]
- Iyanagi T (2007) Molecular mechanism of phase I and phase II drug-metabolizing enzymes: implications for detoxification. Int Rev Cytol 260: 35-112. [Crossref]
- Smith DA, Allerton C, Kalgutkar AS, van de Waterbeemd H, Walker DK (2012) Pharmacokinetics and Metabolism in Drug Design.
- Denisov IG, Makris TM, Sligar SG, Schlichting I (2005) Structure and chemistry of cytochrome P450. Chem Rev 105: 2253-2277. [Crossref]
- Ortiz De Montellano PR (2010) Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem Rev 110: 932-948. [Crossref]
- Sellés Vidal L, Kelly CL, Mordaka PM, Heap JT (2018) Review of NAD(P)H-dependent oxidoreductases: Properties, engineering and application. Biochim Biophys Acta Proteins Proteom. 1866: 327-347. [Crossref]
- Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138: 103-141. [Crossref]
- van der Weide J, Hinrichs JWJ (2006) The influence of cytochrome P450 pharmacogenetics on disposition of common antidepressant and antipsychotic medications. Clin Biochem Rev 27: 17-25. [Crossref]
- Daina A, Michielin O, Zoete V (2017) SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci Rep 7: 42717. [Crossref]
- Guimarães AG, Xavier MA, de Santana MT, Camargo EA, Santos CA et al. (2012) Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn Schmiedebergs Arch Pharmacol 385: 253-263. [Crossref]
- Liang WZ, Lu CH (2012) Carvacrol-induced [Ca2+]i rise and apoptosis in human glioblastoma cells. Life Sci 90: 703-711. [Crossref]
- De Simone R, Ajmone Cat MA, Carnevale D, Minghetti L (2005) Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation 2: 4. [Crossref]
- Hotta M, Nakata R, Katsukawa M, Hori K, Takahashi S et al. (2010) Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J Lipid Res 51: 132-139. [Crossref]
