Carcinoma Showing Thymus-Like Differentiation (CASTLE): A Rare Tumor of the Thyroid
A B S T R A C T
Carcinoma showing thymus-like differentiation (CASTLE) is a rare neoplasm of the thyroid or the adjacent tissues in the neck. It was first described by Miyauchi et al. in 1985 as an intrathyroidal epithelial thymoma. In 1991 Chan and Rosai classified these tumors into four types including CASTLE. World Health Organization (WHO) declared it as an independent clinicopathologic entity in 2004. The tumor arises from ectopic thymus tissue or remnants of branchial pouch. Both sexes are affected similarly with a slight female dominance. It is usually encountered in the fourth and fifth decades of life. It does not have specific symptoms or radiologic findings which makes preoperative diagnosis difficult. It has a higher tendency to be located in the lower poles of thyroid lobes. Immunohistochemistry helps differentiate it from other malignant neoplasms, CD5 being an important marker. The tumor is negative for thyroid specific markers as thyroglobulin, TTF-1 or calcitonin. Surgery is considered the mainstream therapy. Radiotherapy may be reserved for gross disease or recurrence. The role of chemotherapy is unclear. The prognosis of CASTLE is favourable.
Keywords
CASTLE, thyroid carcinoma, thymus-like differentiation, surgery, radiotherapy
Introduction
Carcinoma showing thymus-like differentiation (CASTLE) is a rare neoplasm of the thyroid or the adjacent tissues in the neck. It was first described by Miyauchi et al. in 1985 as an intrathyroidal epithelial thymoma [1]. In 1991 Chan and Rosai classified these tumors into four groups: ectopic hamartomatous thymoma, ectopic cervical thymoma, spindle epithelial tumors with thymus-like differentiation (SETTLE), and carcinoma showing thymus-like elements (CASTLE) [2]. In 2004 World Health Organization (WHO) declared it as an independent clinicopathologic entity [3]. The tumor arises from ectopic thymus tissue within the thyroid or remnants of the branchial pouch [4, 5]. CASTLE exhibits malignant characters. Both sexes are affected similarly with a slight female dominance. It is most common during fourth and fifth decades of life [3]. Preoperative diagnosis is difficult, and patients present with nonspecific symptoms like a slow growing mass in the neck [3]. Imaging of the neck does not have any specific features. Surgery is the mainstay therapy of CASTLE [6, 7]. Patients with locally advanced disease may benefit from radiotherapy. The role of chemotherapy is unclear [7, 8]. The prognosis of CASTLE is favourable [9].
Case Report
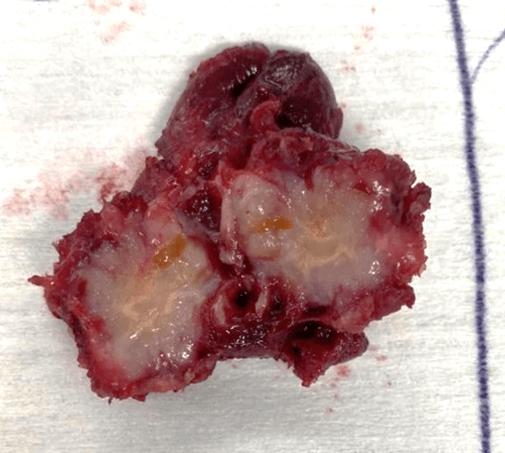
Here, we report a case of CASTLE. A 52-year-old female patient admitted to our clinic for a lump in the neck. She had no prior medical condition. Physical examination revealed a firm mass on the right lobe of the thyroid gland. Thyroid hormone, TSH and thyroglobulin levels were all within normal range. Ultrasonography of the neck showed a thyroid nodule with well-defined borders. FNA of the lesion reported follicular lesion. Bilateral total thyroidectomy was performed. Surrounding muscle tissue seemed to be attacked by the tumor, hence they were removed with the thyroid as well (Figure 1).
Figure 1: Macroscopy.
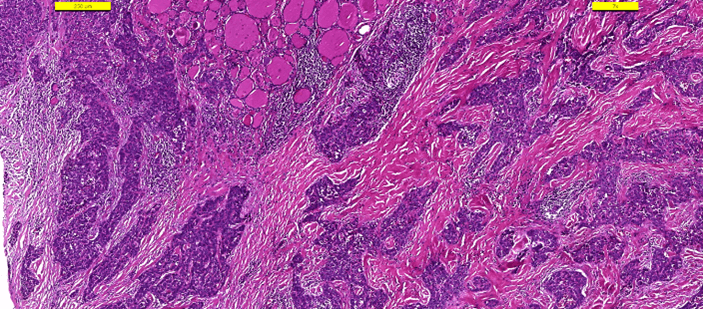
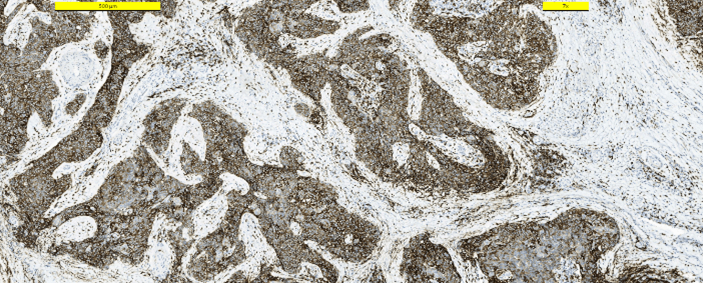
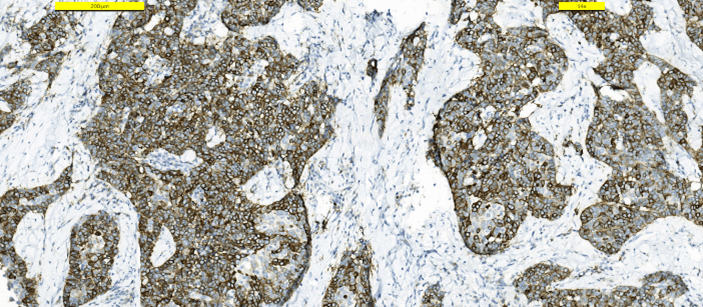
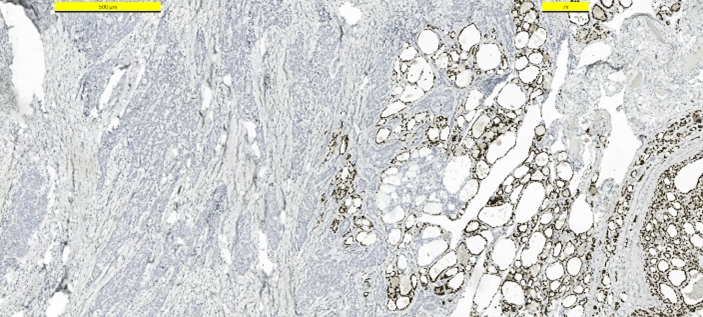
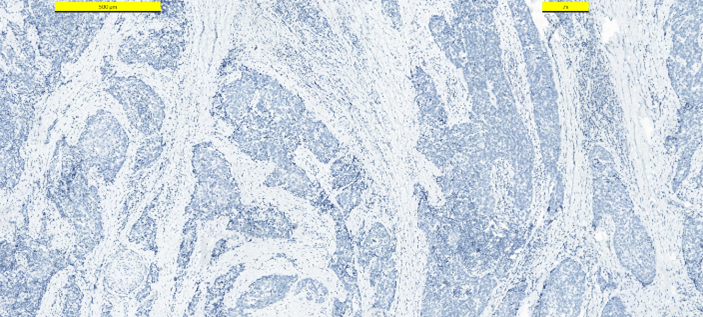
Frozen section examination revealed malignant squamous cells. A distinction between squamous cell carcinoma and thymic cancer could not be made. Postoperative period was uneventful. The patient was discharged from the hospital on the first postoperative day. The patient received no other treatment. Pathologic examination demonstrated a 25 mm intrathyroidal thymic carcinoma (CASTLE) in the right lobe (Figure 2). The tumor had focal infiltration of the surrounding soft and muscle tissue. There was no lymphovascular invasion but wide perineural invasion was seen. Differential diagnosis included medullary thyroid carcinoma, anaplastic carcinoma and squamous cell carcinoma. Immunohistochemistry with adequate controls show that the tumor cells were positive for p40, CD5 (Figure 3), CD117 (Figure 4) and negative for TTF-1 (Figure 5), thyroglobulin, calcitonin (Figure 6) and chromogranin. These findings supported the diagnosis.
Figure 2: Hematoxylin-Eosin.
Figure 3: Immunohistostaining CD5 positive in tumor cells.
Figure 4: Immunohistostaining CD117 positive in tumor cells.
Figure 5: Immunohistostaining TTF-1 positive in thyroid cells, negative in tumor cells.
Figure 6: Immunohistostaining calcitonin negative in tumor cells.
Discussion
Thymus-like tumors are rare tumors that arise from the thyroid or adjacent soft tissues. It accounts for only 0.1-0.15% of all thyroid cancers [10]. It was first described by Miyauchi et al. as intrathyroidal epithelial thymoma in 1985; later in 1994, Chan and Rosai defined these tumors into four classifications: ectopic hamartomatous thymoma, ectopic cervical thymoma, spindle epithelial tumors with thymus-like differentiation (SETTLE), and carcinoma showing thymus-like elements (CASTLE) [1, 2]. In 2004 World Health Organization (WHO) designated it as an independent clinicopathologic entity [3]. The tumor arises from ectopic tissue within thyroid or adjacent soft tissues. It is believed to originate from ectopic thymus tissue or remnants of the branchial pouch [4, 11]. Both sexes are affected similarly with a slight female dominance (F:M=1.3:1). The most common age of presentation is fourth and fifth decades of life although it can be seen in other decades of life [3]. Symptoms usually include a slow-growing, painless neck mass [3]. Hoarseness due to recurrent nerve involvement could be encountered. Dyspnea and dysphagia may result from direct compression of a locally advanced tumor. None of these symptoms are specific to the tumor. Brain, liver, and lung metastasis have been reported; however, they are rare.
Radiologic imaging of the tumor is not specific either [7]. Ultrasonography (US) demonstrates a hypoechoic solid mass of the thyroid without calcifications. Computerized tomography (CT) shows a soft tissue mass of the neck. CT may help see the extent of the mass if it grows towards surrounding as the trachea [12]. Fine needle aspiration biopsy (FNAB) offers limited value in the preoperative management of the patient with most cases being identified as anaplastic carcinoma or poorly differentiated carcinoma. Lack of atypical cytologic features for papillary, follicular, medullary, and undifferentiated carcinoma may lead the pathologist to other diagnosis [13]. Thyroid hormone levels, TSH or thyroglobulin (TG) are within normal range. The tumor tends to be in the lower poles of the thyroid. Neuroendocrine carcinoma/medullary carcinoma, squamous cell carcinoma and anaplastic carcinoma should be considered in differential diagnosis [8]. Histologically prominent features of CASTLE are:
i. Lobulation on cut surfaces.
ii. Expansive growth pattern.
iii. Thick bands within the tumor.
iv. Presence of abundant lymphocytes.
v. Perivascular spaces that contain lymphocytes.
vi. Hassal’s corpuscle-like structures.
vii. Oval, vesicular nuclei.
viii. Well-defined nucleoli and pale cytoplasm.
ix. Low mitotic rate.
x. Absent or limited coagulative necrosis.
xi. Lack of anaplastic, papillary, or follicular tumor.
Immunohistochemistry may help differentiate between these tumors [14]. CD5 is a marker for thymic carcinomas, however not all thymic malignancies express CD5. CD5 and CD117 staining in epithelial cell is highly supportive for CASTLE. CD5 has been reported to be highly specific for CASTLE and is a distinctive feature of it (sensitivity and specificity, 82% and 100%, respectively. Reimann et al. demonstrated that the expression of CD5, HMWCK, CEA and p63 in CASTLE were useful markers to confirm the diagnosis [15]. Tumor cells are also negative for thyroglobulin, calcitonin, and thyroid transcription factor-1, which rule out other primary thyroid neoplasms [11]. Due to limited number of patients, choice of treatment is controversial [6]. Surgical therapy seems to be the main therapy for non-metastatic disease. Surgical resection of the thyroid seems to have favourable results. Central and/or lateral neck dissection is indicated if there is lymph node involvement. CASTLE is a radiosensitive tumor. Roka et al. advocated that surgical resection is sufficient for node negative patients and adjuvant radiotherapy should be reserved for recurrences. Locoregional recurrence rate is 14% after thyroidectomy.
The incidence of extrathyroidal extension is 50% to 60%. In their study, Ito et al. reported a higher rate of nodal metastasis. 50% of patients had nodal metastasis and 29% of them had node metastasis in the lateral neck compartment. So, they suggested to perform modified radical neck dissection not only therapeutically, but also prophylactically. In case of locally advanced disease (extrathyroidal extension or lymph node involvement), radiotherapy has been shown to be beneficial in the local control of the disease and improve survival. In a study conducted by Ito et al., among 22 patients with CASTLE, ten patients -nine had extrathyroidal extension- received radiotherapy. None of the patients who received adjuvant radiotherapy had locoregional recurrence during their follow-up. Locoregional recurrence was seen in three patients who did not receive postoperative radiotherapy. These findings encourage the addition of adjuvant radiotherapy to those patients with gross disease or lymph node metastasis [16]. The absence of nodal metastasis and tumor extension were suggested as indicators of favourable prognosis [13]. The role of chemotherapy is unclear [7, 8]. Chemotherapy has been suggested for patients with metastasis, however, survival benefit has not been shown. Kakudo et al. reported disease progression in one patient while another patient demonstrated complete radiological remission with cisplatin and epirubicin [17]. Prognosis of the tumor is favourable [9]. 5-year and 10-year cause specific survival rates are 90% and 82%, respectively [6]. Surgery can improve the survival of patients. Radiotherapy can be reserved for patients with extrathyroidal extension and lymph node involvement. The treatment methods are debatable due to the rarity of this clinicopathologic entity [10, 18].
Article Info
Article Type
Case ReportPublication history
Received: Wed 01, Dec 2021Accepted: Mon 20, Dec 2021
Published: Mon 27, Dec 2021
Copyright
© 2023 Hakan Kaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.12.05
Author Info
Hakan Kaya Fatma Tokat Saran Duren Burak Ertas
Corresponding Author
Hakan KayaDepartment of Endocrine Surgery, Acıbadem Maslak Hospital, Istanbul, Turkey
Figures & Tables
References
1. Miyauchi A, Kuma K,
Matsuzuka F, Matsubayashi S, Kobayashi A et al. (1985) Intrathyroidal
epithelial thymoma: an entity distinct from squamous cell carcinoma of the
thyroid. World J Surg 9: 128-135. [Crossref]
2.
Chan JK, Rosai J (1991)
Tumors of the neck showing thymic or related branchial pouch differentiation: a
unifying concept. Hum Pathol 22: 349-367. [Crossref]
3.
Liu Z, Teng XY, Sun DX, Xu
WX, Sun SL (2013) Clinical analysis of thyroid carcinoma showing thymus-like
differentiation: report of 8 cases. Int Surg 98: 95-100. [Crossref]
4.
Roka S, Kornek G, Schüller
J, Ortmann E, Feichtinger J et al. (2004) Carcinoma showing thymic-like
elements--a rare malignancy of the thyroid gland. Br J Surg 91: 142-145.
[Crossref]
5.
Abeni
C, Ogliosi C, Rota L, Bertocchi P, Huscher A et al. (2014)
Thyroid carcinoma showing thymus-like differentiation: Case presentation of a
young man. World J Clin Oncol 5: 1117-1120. [Crossref]
6.
Dualim DM, Loo GH, Suhaimi
SNA, Md Latar NH, Muhammad R et al. (2019) The ‘CASTLE’ tumour: An extremely
rare presentation of a thyroid malignancy. A case report. Ann Med Surg (Lond)
44: 57-61. [Crossref]
7.
Fung ACH, Tsang JS, Lang
BHH (2019) Thyroid Carcinoma Showing Thymus-Like Differentiation (CASTLE) with
Tracheal Invasion: A Case Report. Am J Case Rep 20: 1845-1851. [Crossref]
8.
Cappelli
C, Tironi A, Marchetti GP, Pirola I, De Martino E et al. (2008)
Aggressive thyroid carcinoma showing thymic-like differentiation (CASTLE): case
report and review of the literature. Endocr J 55: 685-690. [Crossref]
9.
Okubo Y, Sakai M, Yamazaki
H, Sugawara Y, Samejima J et al. (2020) Histopathological study of carcinoma
showing thymus-like differentiation (CASTLE). Malays J Pathol 42:
259-265. [Crossref]
10. Kong
F, Ying H, Zhai R, Du C, Huang S et al. (2016) Clinical outcome of intensity
modulated radiotherapy for carcinoma showing thymus-like differentiation. Oncotarget
7: 81899-81905. [Crossref]
11. Noh
JM, Ha SY, Ahn YC, Oh D, Seol SW et al. (2015) Potential Role of Adjuvant
Radiation Therapy in Cervical Thymic Neoplasm Involving Thyroid Gland or Neck. Cancer
Res Treat 47: 436-440. [Crossref]
12. Ge
W, Yao YZ, Chen G, Ding YT (2016) Clinical analysis of 82 cases of carcinoma
showing thymus-like differentiation of the thyroid. Oncol Lett 11:
1321-1326. [Crossref]
13. Gao
R, Jia X, Ji T, Feng J, Yang A et al. (2018) Management and Prognostic Factors
for Thyroid Carcinoma Showing Thymus-Like Elements (CASTLE): A Case Series
Study. Front Oncol 8: 477. [Crossref]
14. Zhang
G, Liu X, Huang W, Li X, Johnstone M et al. (2015) Carcinoma showing
thymus-like elements of the thyroid gland: report of three cases including one
case with breast cancer history. Pathol Oncol Res 21: 45-51. [Crossref]
15. Reimann
JDR, Dorfman DM, Nosé V (2006) Carcinoma showing thymus-like differentiation of
the thyroid (CASTLE): a comparative study: evidence of thymic differentiation
and solid cell nest origin. Am J Surg Pathol 30: 994-1001. [Crossref]
16. Lominska
C, Estes CF, Neupane PC, Shnayder Y, TenNapel MJ et al. (2017) CASTLE Thyroid
Tumor: A Case Report and Literature Review. Front Oncol 7: 207. [Crossref]
17. Kakudo K, Bai Y, Ozaki T, Homma K, Ito Y et al. (2013) Intrathyroid epithelial thymoma (ITET) and carcinoma showing thymus-like differentiation (CASTLE): CD5-positive neoplasms mimicking squamous cell carcinoma of the thyroid. Histol Histopathol 28: 543-556. [Crossref]
18. Chan LP, Chiang FY, Lee KW, Kuo WR (2008) Carcinoma showing thymus-like differentiation (CASTLE) of thyroid: a case report and literature review. Kaohsiung J Med Sci 24: 591-597. [Crossref]
