Cadaveric Thoracic Disc Herniation: Fine Architecture of the Prolapse and Relationship with the Posterior Longitudinal Ligament
A B S T R A C T
Prolapse of a lower intervertebral thoracic disc (T10-11) was noticed in a cadaver following examination of serial plastinated sections of the spine. A number of structures were associated with the posteriorly herniated nucleus pulposus, including the posterior longitudinal ligament, fibrous meshworks, venous plexuses and a delicate surrounding capsule. Dimensions of the herniation suggest that the lesion was asymptomatic in life. Thoracic disc prolapse is a rare phenomenon in vivo and is even more infrequently seen in cadavers. This study adds to the minute body of literature on post-mortem thoracic disc herniation and provides insights into detailed pathological changes in the anatomy of surrounding structures following disc prolapse.
Keywords
Thoracic disc prolapse, thoracic disc herniation, posterior longitudinal ligament, cadaver, nucleus pulposus
Introduction
Thoracic disc prolapse (TDP) entails protrusion of the central nucleus pulposus posteriorly out of the encircling annulus fibrosus. The first case of TDP, in the thoracic (T) 12 disc, was described in 1911 by Middleton and Teacher and since then, over 400 cases have been reported [1, 2]. TDP presents extremely infrequently, affecting around 1:1,000,000 people and accounts for 0.15 - 4.0% of all spinal disc herniations, with lumbar and cervical protrusions being four and eight times as prevalent compared to TDP [2-17]. The rarity of TDP is attributed to 1) the comparatively thin discs of the thoracic vertebrae, 2) minimal flexion of the thoracic spine due to the thoracic facets and 3) the ribcage which splints and supports the thoracic spine [4, 18]. Sex bias of this lesion is divided: some authors report that it affects males more than females, others state that it presents in both genders equally and Singounas et al. (1992) describe a preponderance in females [2, 3, 8, 9, 11, 12, 14, 19, 20]. TDP most commonly affects people in their fourth to sixth decade of life [2-4, 8, 9, 14, 19, 20].
Thoracic prolapse is caused by radial fissuring of the annulus fibrosus allowing migration and extrusion of the nucleus pulposus, which often becomes calcified and exhibits surrounding neovascularisation [5, 11, 17-19, 21]. Such a prolapse impinges on both the spinal cord directly and also on the anterior spinal artery. The compression of these structures is amplified by the thoracic kyphosis, the smaller space within the thoracic spinal canal and the paucity of vascularisation compared to the cervical and lumbar regions [4, 8, 9, 18, 19]. In the majority of cases, the protrusion is positioned centrally in the canal and is located in the lower thoracic vertebrae. However, in several instances, T1 or T2 prolapse has been observed [2, 4, 7, 8, 10, 12, 14-16, 20, 22].
In most cases, spinal trauma is not strongly linked to developing TDP. Rather, chronic disc degeneration is indicated in its pathogenesis [2, 3, 7-10, 14, 18, 20, 21]. Patients with TDP can first present with midline back aching, which then may slowly progress to include symptoms such as paraplegia, paraparesis, paraesthesia, radiating pain and visceral disturbances [2-6, 12, 14, 19]. Notwithstanding, most cases of TDP are asymptomatic due to the negligible size of the prolapse [5, 7, 18, 23]. Due to the frequent absence of symptoms or their slow, subtle onset and nonspecific presentation, diagnosis of TDP is very difficult and should be confirmed using radiological imaging such as CT or MRI [3, 4, 8, 18, 24]. Surgical treatment of TDP is difficult: the spinal denticulate ligaments require transection, and the spinal cord frequently needs to be moved aside to access the lesion. Furthermore, adhesions exist between the thoracic disc and the spinal dura and arachnoid therefore, there is a risk of damage to the spinal cord during procedures and subsequent postoperative dysfunction [4, 12, 13, 21]. Laminectomy for TDP is strongly contraindicated, and a lateral surgical approach to the vertebra has been cited as the most optimal in minimising manipulative trauma to the cord and spinal nerves [2-4, 13, 18, 20, 22, 24, 25]. Virtually all reports on TDP are from live case studies. However, there are two studies by Arseni and Nash (1959) and Haley and Perry (1950) that report the presence of TDP in 56 and 11 cadaver specimens, respectively [7, 17].
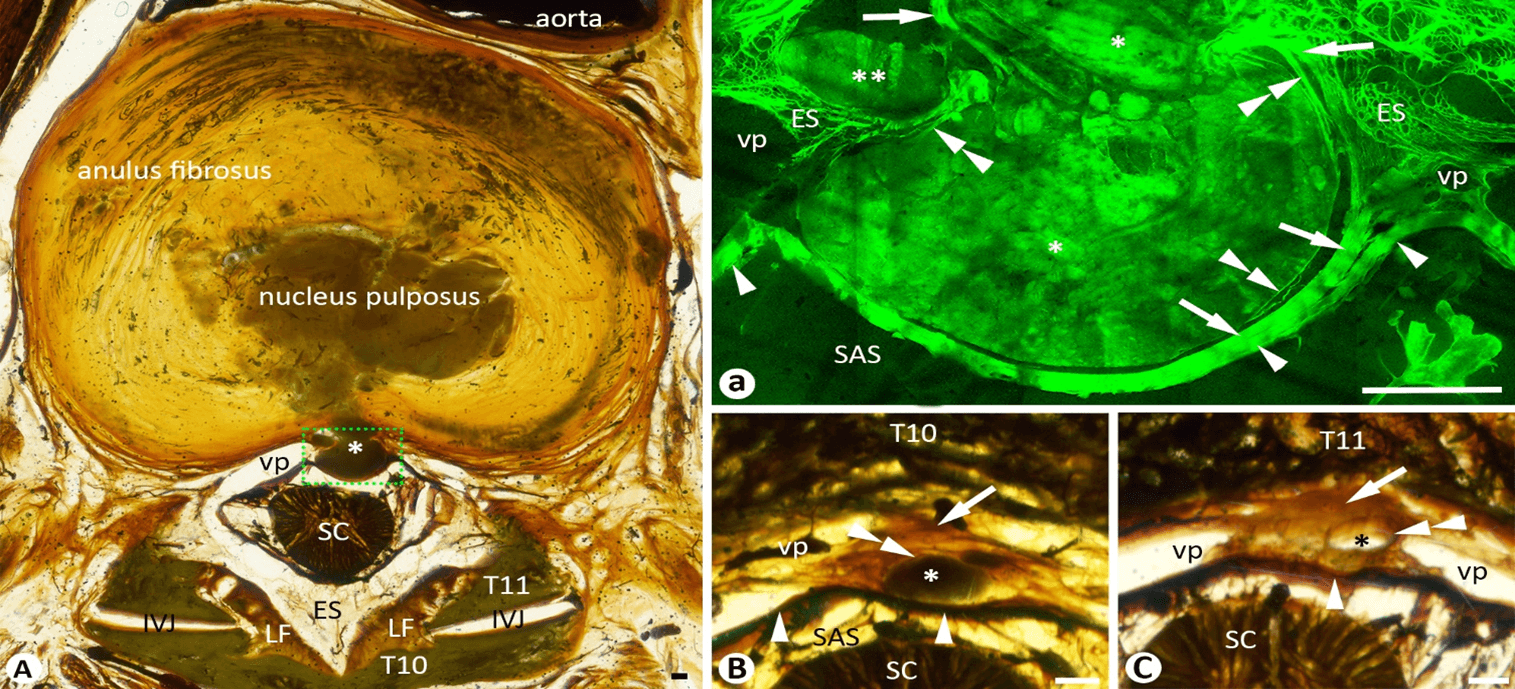
Case Description
An 89-year-old female cadaver who died of acute myocardial infarction was bequeathed for medical education and research purposes under the New Zealand Human Tissue Act (2008). The spine was sliced as a series of 218 transverse sections (thickness of 2.5 mm and an interval of 0.9 mm), which were plastinated as previously described [26]. Upon examination of the plastinated sections, a thoracic disc herniation (TDH) or TDP was incidentally observed at the level of T10-11 (Figure 1A). The nucleus pulposus of the disc herniated through the posterior central fibers of the annulus fibrosus and pushed out the posterior longitudinal ligament (PLL) against the spinal dura forming a forward concaved, thickened PLL/dural arcade (Figures 1A & 1a). The neck of the herniation was surrounded by the fibrous mesh network and venous plexus (Figure 1a). The herniated nucleus was about 10.6% (127 mm3 / 1196 mm3) of the total volume of the nucleus. The main part of the herniated nucleus had a thin capsule that originated from the deep layer of the PLL (Figure 1a). A small portion of the herniation ruptured through the PLL and was surrounded by the mesh network in the epidural space (Figure 1a). The main herniation and its capsule tapered between the PLL and dura around 10.2 mm superiorly up to the level of the middle third of the T10 vertebral body, which had a thickness of 27.2 mm (Figure 1B), and inferiorly about 3.4 mm down to the upper margin of the T11 vertebral body which had a thickness of 30.6 mm (Figure 1C).
Figure 1: A-C) Thoracic disc herniation on a series of cadaveric transverse sections. A) the neck of the herniated nucleus (asterisk) of the T10-11 disc. a) the mirror confocal image of the dashed line box in A, showing parts of the herniated nucleus with (single asterisks) and without (double asterisks) a thin fibrous capsule (double arrowheads) which are surrounded by the fibrous mesh network and venous plexus in the epidural space. Arrows point to the superficial layer of the posterior longitudinal ligament. Single arrowheads point to the spinal dura. B) A transverse section 10.2 mm superior to A. C) A transverse section 3.4 mm inferior to A. Single asterisk: the herniated nucleus; single arrows: posterior longitudinal ligament; double arrowheads: fibrous capsule of the herniation; IVJ: Intervertebral Joint; SC: Spinal Cord; LF: Ligamenta Flava; VP: Venous Plexus; SAS: Subarachnoid Space; Bars = 1 mm.
Discussion
Although it is unknown whether any symptoms accompanied her TDH, the cadaveric case presented in this report was most likely asymptomatic because 1) the herniation was limited by the PLL along the posterior midline, 2) only about 10% of the nucleus was prolapsed, and 3) the spinal cord did not appear to be compressed. Despite TDH having a prevalence of 11-37% in asymptomatic patients and 7-15% in unselected cadavers, very few reports have described the fine fibrous architecture of the TDH and its relationship with the surrounding structures [7, 17, 27-30]. The results of this study indicate that TDH may initiate at the posterior central area of the annulus where the deep layer of the PLL is composed of decussating annulus fibers, and thus the PLL acts as the first barrier against herniation and also limits the extension of the herniation along with the PLL. If the herniated nucleus ruptures the PLL, the TDH may expand laterally and compress the spinal cord and/or spinal nerves, causing symptoms, which may be a possible anatomical mechanism for the TDH to progress from asymptomatic to symptomatic. The composition, strength and extents of the PLL are different along the spine and thus, whether TDH is symptomatic or asymptomatic may also vary amongst individuals and different levels of the thoracic spine [31, 32].
Learning Points
i. Thoracic disc herniation initiates at the posterior central annulus.
ii. The posterior longitudinal ligament acts as the first barrier to limit thoracic disc prolapse.
iii. Symptomatic or asymptomatic thoracic disc herniation may depend on whether the herniation ruptures the barrier of the posterior longitudinal ligament.
Conflicts of Interest
None.
Abbreviation
PLL: Posterior Longitudinal Ligament
T: Thoracic
TDH: Thoracic Disc Herniation
TDP: Thoracic Disc Prolapse
Article Info
Article Type
Case ReportPublication history
Received: Mon 02, Aug 2021Accepted: Thu 26, Aug 2021
Published: Thu 09, Sep 2021
Copyright
© 2023 Ming Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.09.09
Author Info
Han Zhang Jacob Bond Ming Zhang
Corresponding Author
Ming ZhangDepartment of Anatomy, University of Otago, Dunedin, New Zealand
Figures & Tables
References
1. Middleton GS,
Teacher JH (1911) Injury of the Spinal Cord Due to Rupture of an Intervertebral
Disc during Muscular Effort. Glasgow Med
J 76: 1-6. [Crossref]
2. Singounas EG,
Kypriades EM, Kellerman AJ, Garvan N (1992) Thoracic disc herniation. Analysis
of 14 cases and review of the literature. Acta
Neurochir (Wien) 116: 49-52. [Crossref]
3. Carson J, Gumpert
J, Jefferson A (1971) Diagnosis and treatment of thoracic intervertebral disc
protrusions. J Neurol Neurosurg
Psychiatry 34: 68-77. [Crossref]
4. Naylor A (1977)
Surgery in the treatment of cervical and thoracic disc protrusions. Br Med J 1: 821-823. [Crossref]
5. Ryan RW, Lally JF,
Kozic Z (1988) Asymptomatic calcified herniated thoracic disks: CT recognition.
AJNR Am J Neuroradiol 9:
363-366. [Crossref]
6. Gjerris F, Jepsen
BV (1975) Thoracic intervertebral disc herniation. Acta Neurol Scand 52: 395-400. [Crossref]
7. ARSENI C, NASH F
(1960) Thoracic intervertebral disc protrusion: a clinical study. J Neurosurg 17: 418-430. [Crossref]
8. Bury RW, Powell T
(1989) Prolapsed thoracic intervertebral disc: the importance of CT assisted
myelography. Clin Radiol 40: 416-421.
[Crossref]
9. LOGUE V (1952)
Thoracic intervertebral disc prolapse with spinal cord compression. J Neurol Neurosurg Psychiatry 15:
227-241. [Crossref]
10. Davies PR, Kaar G
(1993) High thoracic disc prolapse in a rugby player: the first reported case. Br J Sports Med 27: 177-178. [Crossref]
11. Pai RR, D’sa B,
Raghuveer CV, Kamath A (1999) Neovascularization of nucleus pulposus. A
diagnostic feature of intervertebral disc prolapse. Spine (Phila Pa 1976) 24: 739-741. [Crossref]
12. Singounas EG,
Karvounis PC (1977) Thoracic disc protrusion. (Analysis of 8 cases). Acta Neurochir (Wien) 39:
251-258. [Crossref]
13. Signorini G,
Baldini M, Vivenza C, Princi L, Tonnarelli GP (1979) Surgical treatment of
thoracic disc protrusion. Acta Neurochir
(Wien) 49: 245-254. [Crossref]
14. LOVE JG, SCHORN VG
(1965) THORACIC-DISK PROTRUSIONS. JAMA
191: 627-631. [Crossref]
15. Kumar R, Buckley TF
(1986) First thoracic disc protrusion. Spine
(Phila Pa 1976) 11:
499-501. [Crossref]
16. Hamlyn PJ, Zeital
T, King TT (1991) Protrusion of the first thoracic disk. Surg Neurol 35: 329-331. [Crossref]
17. HALEY JC, PERRY JH
(1950) Protrusions of intervertebral discs: study of their distribution,
characteristics and effects on the nervous system. Am J Surg 80: 394-404. [Crossref]
18. Vanichkachorn JS,
Vaccaro AR (2000) Thoracic disk disease: diagnosis and treatment. J Am Acad Orthop Surg 8: 159-169. [Crossref]
19. Adams MA, Dolan P
(2012) Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat 221: 497-506. [Crossref]
20. Russell T (1989)
Thoracic intervertebral disc protrusion: experience of 67 cases and review of
the literature. Br J Neurosurg 3:
153-160. [Crossref]
21. Reeves DL, Brown HA
(1968) Thoracic intervertebral disc protrusion with spinal cord compression. J Neurosurg 28: 24-28. [Crossref]
22. Kumar R, Cowie RA
(1992) Second thoracic disc protrusion. Spine
(Phila Pa 1976) 17: 120-121. [Crossref]
23. Okada E, Daimon K,
Fujiwara H, Nishiwaki Y, Nojiri K et al. (2019) Ten-year Longitudinal Follow-up
MRI Study of Age-related Changes in Thoracic Intervertebral Discs in
Asymptomatic Subjects. Spine (Phila
Pa 1976) 44: E1317-E1324. [Crossref]
24. Lesoin F, Rousseaux
M, Autricque A, Reesaul Y, Villette L et al. (1986) Thoracic disc herniations:
evolution in the approach and indications. Acta
Neurochir (Wien) 80: 30-34. [Crossref]
25. HULME A (1960) The
surgical approach to thoracic intervertebral disc protrusions. J Neurol Neurosurg Psychiatry 23:
133-137. [Crossref]
26. Nash L, Nicholson
HD, Zhang M (2005) Does the investing layer of the deep cervical fascia exist? Anesthesiololgy 103: 962-968. [Crossref]
27. Awwad EE, Martin
DS, Smith Jr KR, Baker BK (1991) Asymptomatic versus symptomatic herniated
thoracic discs: their frequency and characteristics as detected by computed
tomography after myelography. Neurosurgery
28: 180-186. [Crossref]
28. Gille O, Soderlund
C, Razafimahandri HJC, Mangione P, Vital JM (2006) Analysis of hard thoracic
herniated discs: review of 18 cases operated by thoracoscopy. Eur Spine J 15: 537-542. [Crossref]
29. Williams MP,
Cherryman GR (1988) Thoracic disk herniation: MR imaging. Radiology 167: 874-875. [Crossef]
30. Wood KB, Garvey TA,
Gundry C, Heithoff KB (1995) Magnetic resonance imaging of the thoracic spine.
Evaluation of asymptomatic individuals. J
Bone Joint Surg Am 77: 1631-1638. [Crossref]
31. Loughenbury PR, Wadhwani S, Soames RW (2006) The posterior longitudinal ligament and peridural (epidural) membrane. Clin Anat 19: 487-492. [Crossref]
32. Lee SB, Chang JC, Lee GS, Hwang JC, Bae HG et al. (2018) Morphometric Study of the Lumbar Posterior Longitudinal Ligament. J Korean Neurosurg Soc 61: 89-96. [Crossref]
