BRASH Syndrome: A Case Report and Literature Review
A B S T R A C T
BRASH syndrome is a newly coined diagnosis consisting of the clinical pentad of bradycardia, renal failure, atrioventricular (AV) nodal blockade, shock, and hyperkalemia. It is a rare syndrome with just under 50 reported cases. This case report and literature review present a case of BRASH syndrome, refractory to pharmaceutical measures, and subsequent literature review to assess treatment decisions and overall outcomes. The consensus from the literature supports the use of advanced interventions in most cases, including transcutaneous or transvenous pacing, hemodialysis, and adrenergic support. The early recognition and initiation of treatment in this patient demographic are paramount to reducing possible multi-system organ damage and mortality. This case report and literature review aim to improve patient outcomes and help further elucidate a protocol for treating BRASH syndrome.
Keywords
BRASH syndrome, bradycardia, renal failure, atrioventricular blockade, shock, hyperkalemia
Introduction
The combination of bradycardia, renal failure, atrioventricular (AV) nodal blockade, shock, and hyperkalemia is a recently declared syndrome, described with the acronym BRASH by Farkas et al. in 2016 [1]. This syndrome has only now slowly become recognized in the literature, and, as a result, the clinical significance is not well known. We present a rare case report of BRASH syndrome to bring further awareness to this new diagnosis, including pathophysiology, possible risk factors, and management. We then analyse the literature for cases of BRASH syndrome from 2020 to the present to better understand the utility of advanced support with cardiac pacing, renal replacement therapy, and adrenergic support.
Case Presentation
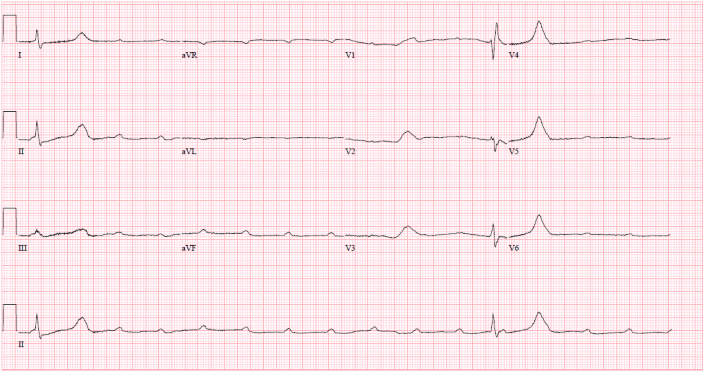
A 66-year-old male with a past medical history of hypertension, hyperlipidemia, chronic obstructive pulmonary disease, alcohol use disorder, mood disorder with psychosis, and multiple previous hospitalizations for drug overdose in the setting of polypharmacy presented to the emergency department with a 3-day history of lethargy and shortness of breath. On presentation, the patient had a blood pressure of 89/55 mmHg, pulse rate of 23, and a Glasgow coma scale (GCS) of 8; he was immediately intubated to protect his airway, and dopamine was initiated. The patient could not provide history; however, his wife reported that he recently refilled his metoprolol tartrate prescription. A subsequent Electrocardiogram (EKG) revealed a third-degree AV block (Figure 1). Despite increasing doses of dopamine, the patient's blood pressure was insufficient, and his heart rate remained in the 30s. Transcutaneous pacing was initiated, followed by the insertion of an internal jugular vein temporary transvenous pacer (TVP).
Labs at the time demonstrated signs of substantial shock, including markedly elevated lactic acid and transaminitis with AST (aspartate transaminase) and ALT (alanine transaminase), both around 1,000 from a previously normal baseline. Additionally, he was found to have new onset acute kidney injury with a creatinine of 3.99 and hyperkalemia at 6.7, requiring hyperkalemic protocol with calcium chloride, insulin, and dextrose. Of note, the patient’s glucose level was euglycemic at 149 mg/dL, making a pure beta blocker overdose unlikely. Troponins peaked at 0.44, and serial EKGs showed no signs of ST changes. Blood and urine cultures and hepatitis B and C and Lyme disease antibody testing were negative. TSH was elevated, but free T4 was within normal range.
His metoprolol was held, and he was given glucagon due to concerns of shock secondary to third-degree AV block from possible beta blocker overdose. The patient was then transferred to the critical care unit, where he was transitioned to norepinephrine. Continuous IV fluids were started, with subsequent labs showing improved signs of end-organ perfusion. After consideration, hemodialysis was not initiated due to adequate urine output.
Figure 1: EKG showing A-V dissociation in third-degree heart block.
Despite normalization of the patient's potassium level, the patient remained in persistent bradycardia requiring continuous TVP, which was upgraded to a permanent pacemaker a few days later. The patient was diagnosed with BRASH syndrome due to the pentad of persistent bradyarrhythmia, acute renal failure, history of AV nodal blocking medication exposure with a beta-blocker, profound hypotension requiring vasopressors, and elevated potassium that required hyperkalemic protocol. The patient ultimately recovered and had an uncomplicated hospital course.
Discussion
The purpose of our case is to highlight the under-recognized diagnosis of BRASH syndrome. BRASH syndrome is a rare constellation of clinical features that includes bradycardia, renal failure, AV nodal blockade, shock, and hyperkalemia [2]. The proposed combination of signs and symptoms is thought to originate with an insult, such as dehydration, that induces prerenal acute kidney injury and associated hyperkalemia [3]. Impaired renal clearance leads to the accumulation of AV nodal blocking agents such as beta-blockers [4]. The synergistic effect of hyperkalemia and increased systemic levels of AV node blockers induce significant bradycardia and decreased cardiac output, leading to signs of shock [5]. Renal hypoperfusion exaggerates the preexisting renal failure, further feeding into the cycle known as BRASH syndrome [6].
The collection of objective clinical findings that compose BRASH syndrome has only recently become recognized, with the diagnosis being first described in 2016 by Farkas et al. [1]. As a result, this disorder's prevalence, management, and overall prognosis are not well known. Various case reports suggest a higher prevalence in the elderly population, especially in those with underlying cardiac and renal dysfunction [2]. It is important for clinicians to maintain a broad differential diagnosis when considering BRASH syndrome, especially since the symptomatology overlaps with other conditions, including medication overdose causing AV node toxicity and secondary causes of hyperkalemia [2]. While an AV nodal blocker overdose can mimic the bradycardia and shock seen in BRASH syndrome, the clinical history can aid in distinguishing the two disease states since patients with BRASH syndrome typically report taking their medications as prescribed [1]. Additionally, measuring the blood sugar level of a patient can be helpful in ruling-out beta-blocker overdose due to the presence of hypoglycemia which is usually seen [2]. Concurrent severe hyperkalemia is often seen in BRASH syndrome and can be distinguished by isolated hyperkalemia due to the lack of typical EKG findings such as peaked T waves and wide QRS complexes [2, 7].
Initial treatment typically includes intravenous (IV) atropine to reverse bradycardia, aggressive IV fluid resuscitation, and correction of acute hyperkalemia. [1]. However, as our case exemplifies, more advanced therapies often need to be initiated in these patients, as this basic treatment approach can fail to both reinstate hemodynamic stability and reverse the end-organ perfusion deficit [1].
Despite the critically ill state that these patients present, there is a substantial gap in knowledge regarding the appropriate clinical management of this condition. To date, less than 50 documented cases of BRASH syndrome have been described in the literature. Due to these patients presenting with acute multi-system organ failure, our goal was to analyse these case reports to understand better the patient outcomes and the overall prognosis of this medical condition. Furthermore, we explored the need to escalate treatment to include three specific aggressive interventions for BRASH syndrome, including temporary transcutaneous or transvenous pacing due to persistent bradyarrhythmias, emergent dialysis for electrolyte imbalances or fluid overload status, and adrenergic medications to maintain systemic perfusion. After an extensive literature search of all case reports from 2020 to the present, we identified 17 cases of documented BRASH syndrome that fulfilled all five components of the diagnosis, including known exposure to an AV-nodal blocking agent [8-21].
We summarize these findings below (Table 1). Overall, the cases we review suggest that BRASH syndrome carries a favourable prognosis, as demonstrated by 82.4% (14/17) of patients being successfully discharged and only three hospital deaths reported. Despite the low mortality rate for a medical condition that involves profound multi-system organ hypoperfusion, aggressive treatment measures to maintain hemodynamic stasis are often needed, such as with our patients. Most of the patients we reviewed (11/17) required adrenergic agonists to maintain systemic perfusion. Dopamine seemed to be the preferred agent used in 6/11 cases, likely due to its favourable combined vasopressor and inotropic effect. While atropine was often the typical initial agent to combat bradycardia, persistent bradycardia was reported in 35.3% (6/17) of cases, which required further intervention with either transcutaneous pacing alone (11.8%) or escalation to transvenous pacing (23.5%). The need for emergent renal replacement therapy was seen in 17.7% (3/17) of patients. Overall, our retrospective review of the literature suggests that timely recognition of BRASH syndrome and appropriate treatment escalation can lead to a promising recovery for patients.
Table 1: Chart review of studies reporting BRASH syndrome
(bradycardia, renal failure, AV nodal blocker, shock, and hyperkalemia) from
2020 to present.
|
Study |
Age (years old) |
Sex |
Atrioventricular-Nodal Blocker Involved |
Bradycardia
Requiring Pacing? |
Renal Failure
Requiring Emergent
Hemodialysis? |
Hemodynamic
Instability Requiring
Adrenergic Agonist? |
Alive at
Discharge? |
|
Gouveia et al. 2022 |
89 |
Female |
Amlodipine |
No |
No |
No |
Yes |
|
Bailuni et al. 2022 |
76 |
Male |
Atenolol
and Amlodipine |
Yes
(transvenous pacing) |
No |
Yes
(Epinephrine) |
Yes |
|
Khan
et al. 2022 |
76 |
Female |
Metoprolol
and Amlodipine |
No |
Yes |
Yes
(Dopamine) |
Yes |
|
Shah
et al. 2022 |
77 |
Female |
Verapamil |
No |
No |
No |
Yes |
|
Shah
et al. 2022 |
86 |
Male |
Metoprolol |
No |
No |
No |
No |
|
Takahashi
et al. 2022 |
86 |
Male |
Carvedilol
and Verapamil |
Yes
(transvenous pacing) |
No |
Yes
(Dopamine) |
Yes |
|
Takahashi
et al. 2022 |
90 |
Female |
Carvedilol
and Amlodipine |
No |
No |
No |
Yes |
|
Ata
et al. 2022 |
64 |
Male |
Bisoprolol |
Yes
(transvenous pacing) |
No |
Yes
(Phenylephrine, Norepinephrine, Vasopressin,
and Dobutamine) |
No |
|
Wong
and Jaafar 2021 |
62 |
Female |
Atenolol
and Diltiazem |
No |
No |
Yes
(Dopamine) |
Yes |
|
Wong
and Jaafar 2021 |
44 |
Female |
Metoprolol,
Diltiazem, and Felodipine |
Yes
(transcutaneous pacing) |
No |
Yes
(Dopamine) |
Yes |
|
Park
et al. 2021 |
71 |
Male |
Amlodipine,
Nifedipine, and Carvedilol |
No |
No |
Yes
(Isoproterenol) |
Yes |
|
Ghumman
et al. 2021 |
69 |
Male |
Metoprolol
succinate and Labetalol |
No |
No |
Yes
(Epinephrine) |
No |
|
Sattar
et al. 2020 |
66 |
Female |
Carvedilol |
No |
No |
No |
Yes |
|
Srivastava et al. 2020 |
62 |
Female |
Carvedilol |
No |
No |
Yes
(Dopamine) |
Yes |
|
Prabhu et al. 2020 |
N/A |
Female |
Carvedilol
and Verapamil |
No |
No |
No |
Yes |
|
Arif et al. 2020 |
55 |
Female |
Diltiazem |
Yes
(transcutaneous pacing) |
Yes |
Yes
(Dopamine) |
Yes |
|
Grigorov et al. 2020 |
43 |
Female |
Metoprolol
tartrate and Diltiazem |
Yes
(transvenous pacing) |
Yes |
Yes
(Norepinephrine) |
Yes |
|
Total |
6/17 |
3/17 (17.6%) |
11/17 |
14/17 |
|||
Conclusion
Our case aims to bring further awareness to the rare combination of clinical features, along with the complex pathophysiology, that underlies BRASH syndrome. After reviewing all currently documented case reports, it is clear the rapid progression towards multi-system organ failure that is often seen in this patient demographic. Our analysis of the literature points to the need for clinicians to be proactive in starting measures to maintain hemodynamic stability including manual mechanical pacing, hemodialysis, or medications for vasopressor or inotropic support. Ultimately, we hope that our contribution to the knowledge of BRASH syndrome may lead to improved patient outcomes and a more robust treatment protocol in the future.
Conflicts of Interest
None.
Funding
None.
Acknowledgment
Not applicable.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Thu 01, Sep 2022Accepted: Tue 20, Sep 2022
Published: Fri 30, Sep 2022
Copyright
© 2023 Steven Imburgio. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2022.03.05
Author Info
Steven Imburgio Anmol Johal Ndausung Udongwo Anton Mararenko Anas Alrefaee Joseph Heaton
Corresponding Author
Steven ImburgioJersey Shore University Medical Center, Department of Medicine, Neptune City, New Jersey, USA
Figures & Tables
Table 1: Chart review of studies reporting BRASH syndrome
(bradycardia, renal failure, AV nodal blocker, shock, and hyperkalemia) from
2020 to present.
|
Study |
Age (years old) |
Sex |
Atrioventricular-Nodal Blocker Involved |
Bradycardia
Requiring Pacing? |
Renal Failure
Requiring Emergent
Hemodialysis? |
Hemodynamic
Instability Requiring
Adrenergic Agonist? |
Alive at
Discharge? |
|
Gouveia et al. 2022 |
89 |
Female |
Amlodipine |
No |
No |
No |
Yes |
|
Bailuni et al. 2022 |
76 |
Male |
Atenolol
and Amlodipine |
Yes
(transvenous pacing) |
No |
Yes
(Epinephrine) |
Yes |
|
Khan
et al. 2022 |
76 |
Female |
Metoprolol
and Amlodipine |
No |
Yes |
Yes
(Dopamine) |
Yes |
|
Shah
et al. 2022 |
77 |
Female |
Verapamil |
No |
No |
No |
Yes |
|
Shah
et al. 2022 |
86 |
Male |
Metoprolol |
No |
No |
No |
No |
|
Takahashi
et al. 2022 |
86 |
Male |
Carvedilol
and Verapamil |
Yes
(transvenous pacing) |
No |
Yes
(Dopamine) |
Yes |
|
Takahashi
et al. 2022 |
90 |
Female |
Carvedilol
and Amlodipine |
No |
No |
No |
Yes |
|
Ata
et al. 2022 |
64 |
Male |
Bisoprolol |
Yes
(transvenous pacing) |
No |
Yes
(Phenylephrine, Norepinephrine, Vasopressin,
and Dobutamine) |
No |
|
Wong
and Jaafar 2021 |
62 |
Female |
Atenolol
and Diltiazem |
No |
No |
Yes
(Dopamine) |
Yes |
|
Wong
and Jaafar 2021 |
44 |
Female |
Metoprolol,
Diltiazem, and Felodipine |
Yes
(transcutaneous pacing) |
No |
Yes
(Dopamine) |
Yes |
|
Park
et al. 2021 |
71 |
Male |
Amlodipine,
Nifedipine, and Carvedilol |
No |
No |
Yes
(Isoproterenol) |
Yes |
|
Ghumman
et al. 2021 |
69 |
Male |
Metoprolol
succinate and Labetalol |
No |
No |
Yes
(Epinephrine) |
No |
|
Sattar
et al. 2020 |
66 |
Female |
Carvedilol |
No |
No |
No |
Yes |
|
Srivastava et al. 2020 |
62 |
Female |
Carvedilol |
No |
No |
Yes
(Dopamine) |
Yes |
|
Prabhu et al. 2020 |
N/A |
Female |
Carvedilol
and Verapamil |
No |
No |
No |
Yes |
|
Arif et al. 2020 |
55 |
Female |
Diltiazem |
Yes
(transcutaneous pacing) |
Yes |
Yes
(Dopamine) |
Yes |
|
Grigorov et al. 2020 |
43 |
Female |
Metoprolol
tartrate and Diltiazem |
Yes
(transvenous pacing) |
Yes |
Yes
(Norepinephrine) |
Yes |
|
Total |
6/17 |
3/17 (17.6%) |
11/17 |
14/17 |
|||
References
1. Farkas JD, Long B,
Koyfman A, Menson K (2020) BRASH Syndrome: Bradycardia, Renal Failure, AV
Blockade, Shock, and Hyperkalemia. J Emerg Med 59: 216-223. [Crossref]
2. Lizyness K, Dewald
O (2022) BRASH Syndrome. StatPearls [Internet]. Treasure Island (FL):
StatPearls.
3. Diribe N, Le J
(2019) Trimethoprim/Sulfamethoxazole-Induced Bradycardia, Renal Failure,
AV-Node Blockers, Shock and Hyperkalemia Syndrome. Clin Pract Cases Emerg
Med 3: 282-285. [Crossref]
4. Pata R, Lutaya I,
Mefford M, Arora A, Nway N (2022) Urinary Tract Infection Causing Bradycardia,
Renal Failure, Atrioventricular Nodal Blockade, Shock, and Hyperkalemia (BRASH)
Syndrome: A Case Report and a Brief Review of the Literature. Cureus 14:
e27641. [Crossref]
5. Graudins A, Lee HM,
Druda D (2016) Calcium channel antagonist and beta-blocker overdose: antidotes
and adjunct therapies. Br J Clin Pharmacol 81: 453-461. [Crossref]
6. Zaidi SAA, Shaikh
D, Saad M, Vittorio TJ (2019) Ranolazine Induced Bradycardia, Renal Failure,
and Hyperkalemia: A BRASH Syndrome Variant. Case Rep Med 2019: 2740617.
[Crossref]
7. Simon LV, Hashmi
MF, Farrell MW (2022) Hyperkalemia. StatPearls [Internet]. Treasure Island
(FL): StatPearls
8. Gouveia R, Veiga H,
Costa AA, Pereira J, Lourenço P (2022) Bradycardia, Renal Failure,
Atrioventricular Nodal Blockade, Shock, and Hyperkalemia Syndrome due to
Amlodipine: A Case Report of an Underdiagnosed Medical Condition. Cureus
14: e21144. [Crossref]
9. Neto JJB, de Lima
Siqueira B, Machado FC, Boros GAB, Akamine MAV et al. (2022) BRASH Syndrome: A
Case Report. Am J Case Rep 23: e934600. [Crossref]
10. Khan A, Lahmar A,
Ehtesham M, Riasat M, Haseeb M (2022) Bradycardia, Renal Failure,
Atrioventricular-Nodal Blockade, Shock, and Hyperkalemia Syndrome: A Case
Report. Cureus 14: e23486. [Crossref]
11. Shah P, Silangruz
K, Lee E, Nishimura Y (2022) Two Cases of BRASH Syndrome: A Diagnostic
Challenge. Eur J Case Rep Intern Med 9: 003314. [Crossref]
12. Takahashi K, Sakaue
T, Uemura S, Okura T, Ikeda S (2022) Bradycardia, Renal Failure,
Atrioventricular Nodal Blockade, Shock, and Hyperkalemia Syndrome as a Clinical
Profile Leading to the Diagnosis of Transthyretin Amyloidosis: A Report of Two
Cases. Cureus 14: e25444. [Crossref]
13. Fateen A, Muhammad
Y, Saad Y, Bint I BA, Bassam M et al. (2021) Diagnostic and therapeutic
challenges of BRASH syndrome: A case report. Med Case Rep Study Protoc
2: e0018.
14. Wong CK, Jaafar MJ
(2021) Bradycardia, renal failure, atrioventricular nodal blockade, shock, and
hyperkalemia: An important syndrome to recognize. Turk J Emerg Med 21:
86-89. [Crossref]
15. Park JI, Jung MS,
Lee H, Kim H, Oh J (2022) Implication of AV node blockers in patients with
end-stage renal disease undergoing head and neck surgery; BRASH syndrome: a
case report. Braz J Anesthesiol 72: 152-155. [Crossref]
16. Ghumman GM, Kumar A
(2021) BRASH Syndrome Leading to Cardiac Arrest and Diffuse Anoxic Brain
Injury: An Underdiagnosed Entity. Cureus 13: e18628. [Crossref]
17. Sattar Y, Bareeqa
SB, Rauf H, Ullah W, Alraies MC (2020) Bradycardia, Renal Failure,
Atrioventricular-nodal Blocker, Shock, and Hyperkalemia Syndrome Diagnosis and
Literature Review. Cureus 12: e6985. [Crossref]
18. Srivastava S,
Kemnic T, Hildebrandt KR (2020) BRASH syndrome. BMJ Case Rep 13:
e233825. [Crossref]
19. Prabhu V, Hsu E,
Lestin S, Soltanianzadeh Y, Hadi S (2020) Bradycardia, Renal Failure,
Atrioventricular Nodal Blockade, Shock, and Hyperkalemia (BRASH) Syndrome as a
Presentation of Coronavirus Disease 2019. Cureus 12: e7816. [Crossref]
20. Arif AW, Khan MS, Masri A, Mba B, Ayub MT et al. (2020) BRASH Syndrome with Hyperkalemia: An Under-Recognized Clinical Condition. Methodist Debakey Cardiovasc J 16: 241-244. [Crossref]
21. Grigorov MV, Belur AD, Otero D, Chaudhary S, Grigorov E et al. (2020) The BRASH syndrome, a synergistic arrhythmia phenomenon. Proc (Bayl Univ Med Cent) 33: 668-670. [Crossref]
