Journals
Benign Lesions Mimicking Local Recurrence after Lung Cancer Segmentectomy
A B S T R A C T
We report two cases of benign lesions mimicking local recurrence after lung cancer segmentectomy. Positron emission tomography (PET) and computed tomography (CT) revealed new nodules with abnormal uptake in the remaining lobes; they were found to be benign lesions. In the first case, the new nodule reduced during the clinical course of the patient. In the second case, completion lobectomy was performed as the intraoperative frozen section diagnosis was malignancy three years after the segmentectomy. These cases suggest that oncologist should consider the possibility of benign lesions mimicking local recurrence in the remaining lobes after segmentectomy for lung cancer.
Case Presentation
The number of radical and anatomical pulmonary segmentectomies performed in cases of early lung cancers with small tumor sizes has been increasing recently in Japan [1, 2]. Segmentectomy is useful for preserving respiratory function; however, the rate of local recurrence of lung cancer is higher for segmentectomy than for lobectomy [3]. The detection of new solitary pulmonary nodules during follow-up after a surgery for lung cancer, especially after limited resection, poses a diagnostic challenge. Positron emission tomography (PET) and computed tomography (CT) is used extensively for the clinical characterization of solitary pulmonary nodules and to differentiate between malignant and benign lesions [4]. However, PET-CT is associated with a high false positive rate in cases of inflammatory lesions, which can lead to unnecessary surgical treatment [5]. We report the cases of two patients with lesions mimicking local recurrence with abnormal uptake on PET after segmentectomy for primary lung carcinomas.
Case Report
Patient 1
A 69-year-old woman underwent right medial basal segment (segment 8) segmentectomy and node dissection for T1aN0M0 adenocarcinoma visualized as a pure ground-glass opacity (GGO) in a preoperative CT scan. Three years post-surgery, a new solitary pulmonary nodule with a maximum standardized uptake value (SUV max) of 9.1 on PET appeared in the remaining lobe (segment 7), and local recurrence was suspected (Fig. 1). The patient refused additional treatment. The nodule reduced during her clinical course without any suspicion of recurrence. She has been in relapse-free survival for eight years.
Patient 2
A 62-year-old woman underwent right anterior segment (segment 2) segmentectomy and node dissection for atypical adenomatous hyperplasia visualized as a pure GGO in a preoperative CT scan. Six years post-surgery, a new solitary pulmonary nodule with an SUV max of 4.5 on PET appeared near the margin in the remaining lobe and was suspected to be a local recurrence (Fig. 2). The patient underwent completion right upper lobectomy because the intraoperative frozen section diagnosis was adenocarcinoma; however, the final pathological diagnosis was epithelioid granuloma with necrosis, with no positive reaction in the acid fast bacteria stain and a negative mycology culture. Her postoperative course was favorable without recurrence.
Comment
The present standard surgical procedure for lung cancer is pulmonary lobectomy with hilar and mediastinal node dissection, based on reports indicating that patients treated with limited resection for cT1N0M0 peripheral lung cancer had a worse prognosis than those treated with pulmonary lobectomy and lymph node dissection [3]. On the other hand, a prospective study reported no recurrence of the primary lung cancer during a 5-year follow-up after limited resection of lung cancer with a maximum tumor diameter of 8–20 mm, a GGO ratio >80% in a CT scan, and clinical T1N0M0; moreover, the 5-year disease-specific and overall survival rates were 100% and 98%, respectively [1]. At our institution, radical and anatomical pulmonary segmentectomies in cases of lung cancers are performed for pulmonary nodules with diameters < 20 mm and GGO ratios >50% in a CT scan. In the two cases presented herein, segmentectomy was performed as both lesions were T1N0M0 lung cancers visualized as pure GGOs in a CT scan before the first surgery.
The first postoperative screening using a CT scan in patients with lung cancers at our institution is performed 6 months post-surgery and at 1-year intervals thereafter. When new solitary pulmonary nodules appear during follow-up, PET is used for the clinical differentiation between malignant and benign lesions [4]. However, other inflammatory lesions can show abnormal uptake on PET, and suture as well as stapler granulomas with abnormal uptake on PET were detected during the follow-up of patients who had undergone a lobectomy and wedge resection for lung cancer [6]. In this case report, new solitary pulmonary nodules with abnormal uptake on PET appeared in the remaining lobes after the segmentectomies. The differential diagnosis included local recurrence, metachronous primary lung cancer and benign inflammatory lesions.
In the case of patient 2, the final histopathological image of the lesion after completion lobectomy was epithelioid granuloma and Langerhans giant cells, caseous necrosis, and lymphocytic infiltrate (Fig. 3). This is the typical histopathological image of tuberculosis and atypical mycobacterial disease, but the acid-fast bacteria stain was negative. It is conceivable that the inflammation image with the lymphocytic infiltrate showed the abnormal uptake on PET. We included atypical mycobacterial disease and granuloma caused by an inflammatory response to the sutures and stapler used in the first surgery in the differential diagnosis. A mycobacterial culture of the patient’s sputum sample should have been examined before the surgery to diagnose or rule out tuberculosis and atypical mycobacterial disease; however, this was not done in this case because we did not suspect these conditions based on the non-inflammatory reaction in the blood chemistry. Granulomas caused by an inflammatory response to stapler lines and tissue degeneration with an energy device occur frequently during segmentectomies because of the resection between lung segments.
An intraoperative frozen section diagnosis is recommended to decide which surgical treatment is needed for lesions suspected to be recurrence after surgeries for lung cancer. In the case of patient 2, the intraoperative frozen section diagnosis was malignancy as the needle biopsy of the tissue samples revealed cells with larger nuclei, dark-stained chromatin, and a high nucleo-cytoplasmic ratio. The final pathological diagnosis however was epithelioid granuloma with necrosis. The atypical cells that had been suspected as carcinoma cells in the intraoperative frozen section diagnosis were in fact enlarged alveolar epithelial cells due to inflammation or bronchiole cells crushed by needle biopsy. Completion lobectomy is extremely difficult, especially after a segmentectomy. Nevertheless, it must be performed when the intraoperative frozen section indicates a malignancy during surgeries for lesions suspected to be local recurrence. It is possible that an intraoperative frozen section diagnosis is more accurate for wedge resection tissue samples.
In conclusion, our report suggests that oncologist should consider the possibility of benign lesions showing abnormal uptake on PET and mimicking local recurrence in the remaining lobes after segmentectomy for lung cancer. Further studies should establish the optimal diagnosis and treatment for new solitary pulmonary nodules suspected of local recurrence after segmentectomies in cases of lung cancers.
Figure 1: Computed tomography scan showing a solitary pulmonary nodule in the remaining lobe (segment 7) after right medial basal segment (segment 8) segmentectomy (A) and abnormal uptake on positron emission tomography (PET), with a maximum standardized uptake value (SUV max) of 9.1 (B).
Figure 2: Computed tomography scan showing a solitary pulmonary nodule near the margin in the remaining lobe after right anterior segment (segment 2) segmentectomy (A) and abnormal uptake on positron emission tomography (PET), with a maximum standardized uptake value (SUV max) of 4.5 (B)
Figure 3: Epithelioid granuloma and Langerhans giant cells, caseous necrosis, and lymphocytic infiltrate (yellow arrows). Hematoxylin & eosin staining, magnification × 10.
Disclosures
The authors declare that they have no conflicts of interest. Ethical approval was not required for a case report. The patient gave informed consent before being included in this report.
Article Info
Article Type
Case ReportPublication history
Received: Wed 11, Apr 2018Accepted: Wed 25, Apr 2018
Published: Fri 04, May 2018
Copyright
© 2023 Atsushi Watanabe. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2018.10.008
Author Info
Atsushi Watanabe Makoto Tada Masahiro Miyajima Mitsuhiro Tsujiwaki Ryunosuke Maki Taijiro Mishina Yuki Takahashi
Corresponding Author
Atsushi WatanabeDepartment of Thoracic Surgery, Sapporo Medical University, School of Medicine and Hospital, South 1, West 16, Chuo-ku, Sapporo, Hokkaido, Japan
Figures & Tables

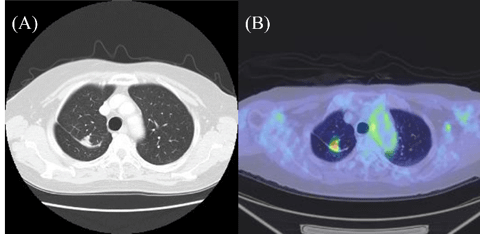

Figure legends
Fig. 1: Computed tomography scan showing a solitary pulmonary nodule in the remaining lobe (segment 7) after right medial basal segment (segment 8) segmentectomy (A) and abnormal uptake on positron emission tomography (PET), with a maximum standardized uptake value (SUV max) of 9.1 (B).
Fig. 2: Computed tomography scan showing a solitary pulmonary nodule near the margin in the remaining lobe after right anterior segment (segment 2) segmentectomy (A) and abnormal uptake on positron emission tomography (PET), with a maximum standardized uptake value (SUV max) of 4.5 (B)
Fig. 3: Epithelioid granuloma and Langerhans giant cells, caseous necrosis, and lymphocytic infiltrate (yellow arrows). Hematoxylin & eosin staining, magnification × 10.
References
1. Sagawa M, Oizumi H, Suzuki H, Uramoto H, Usuda K, et al. (2018) A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 53: 849-856. [Crossref]
2. Nomori H (2014) Segmentectomy for c-T1N0M0 non-small cell lung cancer. Surg Today 44: 812-819. [Crossref]
3. Ginsberg RJ, Rubinstein LV (1995) Randomized trial of lobectomy versus limited resection for T1N0 non-small cell cancer. Ann Thorac Surg 60: 615-622. [Crossref]
4. Hain SF, Curran KM, Beggs AD, Fogelman I, O’Doherty MJ, et al. (2001) FDG-PET as a `metabolic biopsy’ tool in thoracic lesion with indeterminate biopsy. Eur J Clin Med 28: 1336-1340. [Crossref]
5. Low SY, Eng P, Keng GH, Ng DC (2006) Positron emission tomography with CT in the evaluation of non-small cell lung cancer in populations with a high prevalence of tuberculosis. Respirology 11: 84-89. [Crossref]
6. Yüksel MAkgül AG Evman S, Batirel HF (2007) Suture and stapler granulomas: a word of caution. Eur J Cardiothorac Sur 31: 563-565. [Crossref]
