BDE-209 and TCDD Modulate the Expression and Activity of ATP-Binding Cassette (ABC) Transporters in Murine Melanoma Cells (B16-F1)
A B S T R A C T
Persistent organic pollutants (POPs) may alter tumor cells phenotype, possibly increasing malignancy, but there is a lack of studies investigating the mechanisms by which POPs may affect tumor cells. The ATP-Binding Cassette (ABC) transporter proteins are a widely studied component of drug resistance and tumor progression. We hypothesized that the levels of BDE-209 and TCDD detected in human serum can modulate the gene expression or activity of ATP-binding cassette (ABC) transporters in murine melanoma (B16-F1) cells. In this study, we observed an upregulation of the ABCB1 and ABCC4 (24 h) genes followed by an increased protein activity after BDE-209 15 day-exposure. We also observed that cells exposed to TCDD showed an upregulation of ABCB5, ABCC1 and ABCC4 genes (24 h) and change of protein activity after 15 days of exposure. These findings suggest that BDE-209 and TCDD can regulate the phenotype of B16-F1 cells by interfering with the expression and activity of ATP-binding cassette (ABC) transporters. This investigation revealed that environmental pollutants might intervene and modify cells’ resistance to chemotherapy and cancer prognosis.
Keywords
Dioxin, BDE-209, ATP-binding cassette, melanoma, B16-F1
Graphical Abstract
Introduction
Melanoma originates from the transformation of melanocytes and leads to high mortality due to its aggressiveness and a low response to treatments [1, 2]. The overexpression of ATP-dependent drug efflux pumps belonging to the ABC superfamily (ATP-binding cassette) is characterized by MDR (multidrug resistance) phenotype [3, 4]. The role of ABC system is to direct the flux of different substrates through membranes, including anticancer drugs and environmental xenobiotics. These proteins are structurally grouped into two transmembrane regions (TMDs) that bind and translocate different substrates, and two nucleotide ATP binding domains (NBDs) [5]. Two subfamilies of ABC transporters are particularly important in melanoma: ABCB and ABCC. The genes ABCB1, ABCB5, ABCC1, ABCC2 and ABCC4 code for PGP1, also denominated MDR1, while PGP5 (MDR5), MRP1, MRP2 and MRP4 proteins confer resistance to multiple drugs in normal and melanoma cells [6, 7]. Recently, the ABC transport proteins were suggested to be involved in the initiation and progression of tumors [8].
According to Houghton and Polsky, environmental factors as sunlight and chemical exposure favour the development of melanoma [9]. BDE-209 and TCDD are present in domestic dust and high-fat food like eggs, milk, and meat. These chemicals are persistent in the environment and are lipophilic, accumulating in fat tissues [10]. Studies have described the effects of chronic exposure to pollutants, even if at very low concentrations, affecting an array of biological systems [11, 12]. In the last decades, BDE-209 was widely used as a flame retardant in electronics, plastics and an assortment of household products [13, 14]. The chlorinated organic compound 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is considered the most toxic member of the dioxin superfamily [15]. The TCDD has its origin from by-products of various industrial chemical reactions and burn of solid waste as electronic material [16]. Like BDE-209, TCDD is persistent in the environment, lipophilic and bioaccumulates in the food chain [17]. These compounds have been found in foods with high-fat contents, breast milk and even in intrauterine development [18-20]. Despite carcinogenic effects being well-described for various chemicals, our group published the first in vivo study correlating POPs with the progression and increased malignancy of an established type of cancer [21]. In the current study, we investigated the role of TCDD and BDE-209 in the modulation of ABC transporter proteins in murine melanoma cells (B16-F1) after acute and chronic exposure at concentrations previously found in human plasma.
Materials and Methods
I Experimental Design
Stock solutions of Decabromodiphenyl ether (BDE-209) (Sigma Aldrich) and Tetrachlorodibenzo-p-dioxin (TCDD) (Ultra USA) were prepared in dimethyl sulfoxide (DMSO) and kept at -20 °C. Murine melanoma B16-F1 cells (Rio de Janeiro Cell Bank, Brazil) were cultured in high glucose DMEM medium supplemented with 10% fetal bovine serum (FBS) and 40 μg/mL of the antibiotic gentamicin, at 37° C, with 5% CO2. The cells were exposed to TCDD and BDE-209 for 24h and 15 days at concentrations 0.01, 0.1 and 1.0 nM for both contaminants. These concentrations were chosen to pair the levels found in human serum: TCDD at 0.1 nM in blood serum (ATSDR, 1988); BDE-209 at 1 nM in maternal serum [22-24]. For the acute experiment, 6×103 cells/well (30,000 cells/mL) were cultured in 96-well microplates for 24 h for attachment and then exposed to BDE-209 and TCDD in culture medium supplemented with FBS and gentamicin for 24 h. For the chronic experiment, 1×105 cells (10,000 cells/mL) were cultured in 75 cm2 culture flasks as described above and then exposed to BDE-209 and TCDD in culture medium supplemented with FBS and antibiotic. For the chronic exposure, the culture medium was replaced every 72 h, maintaining the original concentration of BDE-209 or TCDD. After 15 days, the cells were detached from culture flasks with trypsin-EDTA and cultured in 96-well microplates for 24 h before the analysis. Control groups were cultured with 0.1% DMSO in culture medium supplemented with FBS and antibiotic.
II Neutral Red Assay
Cells’ viability was evaluated according to Repeto et al., immediately after the acute or chronic experiments [25]. Neutral red assay evaluates the integrity and functionality of the endo-lysosomal system.
III Primer Design, Total RNA Extraction and cDNA Conversion
The primers were designed using the software Primer 3 and Gene Runner with posterior validation by BLASTx as follow: β 2-Microglobulin (amplicon 110bp) (F 5´ CGAGACATGTGATCAAGCATC 3´, R 5´ GCTATTTCTTTCTGCGTGCAT 3´), ABCB1 (amplicon 116bp) (F 5´CATTGCTGGTTTTGATGGTG 3´, R 5´ CAATTTCATTTCCTGCTGTCTG 3´), ABCB5 (amplicon 104bp) (F 5´ CTTTATGAATGGAGCCTA 3´, R 5´ TCACACTGAAGAAAACAGCAAGA 3´), ABCC1 (amplicon 103bp) (F 5´ GAACCTCCCACACTGAATGG 3´, R 5´ GGGCTGACAGCAGAGATGAC 3´), ABCC2 (amplicon 101bp) (F 5´ GCCTCTTTTACTTGGGACC 3´, R 5´AGCCTACAGTGCCCACCACAG 3´) and ABCC4 (amplicon 117bp) (F 5´ GGCTTCAAGGCCTACAAGAA 3´, R 5´ AAGCCACCAGTCCTGAAGAA 3´) where 6 pmol was used per 5 µM of cDNA. The extraction of total RNA was performed using the Purelink RNA Mini Kit (Applied Biosystems - Thermo Fisher) and TRIzol® (Life Technologies) at a ratio of 1:1. The total RNA was quantified in spectrophotometer through the A260/A280 ratio and converted to total cDNA using Superscript IV kit (Applied Biosystems - Thermo Fisher).
IV Quantitative Real-Time PCR
The reactions were performed in a StepOne fast system thermal cycler (Applied Biosystems) using the SYBRGreen PCR Master Mix for each reaction. Thermocycling was performed according to the manufacturer’s recommendations (40 cycles, denaturing at 94°C followed by hybridization at 60°C). The relative quantification value for each target gene was normalized with the endogenous control (beta-2 microglobulin).
V Drug-Efflux Transporters
Rhodamine B and calcein AM, were used to evaluate the activity of the ATP-binding cassette transporters. After exposure, the attached cells were incubated with rhodamine B (1.0 μM, 30 min, 37°C) or Calcein AM (0.5 μM, 60 min, 37°C) and washed with PBS. The procedure was performed according to Liebel et al. and Vernhet et al. [26, 27].
VI Statistical Analysis
Three independent experiments were performed for all the endpoints. For qPCR analysis, the target mRNA levels were normalized to endogenous control (beta-2 microglobulin) by ∆∆CT already included in the thermal cycler software. The statistical analysis included multiple comparisons, and comparisons only between 0.1% DMSO media control and other concentrations. The data were evaluated by Kruskal-Wallis post-test or Dunn’s post-test when applicable, using GraphPad Prism 5 software.
Results
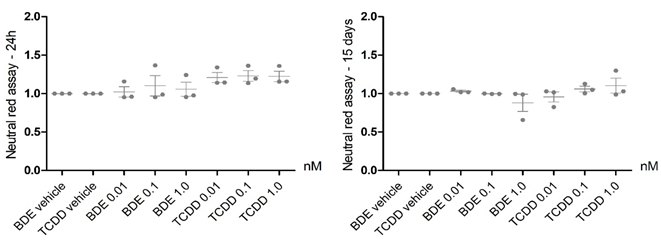
Exposure to BDE-209 and TCDD did not affect the murine melanoma cell B16-F1 viability (Figure 1). Acute exposure to BDE-209 and TCDD lead to alterations in the ABC genes expression. For BDE-209 acute exposure, the ABCB1 gene was overexpressed when compared to the control group (1.0 nM - 20%, p <0.08, Figure 2A); the ABCC4 gene was also overexpressed at the highest concentration when compared with the intermediate concentration (0.1 nM) by 37% and with the control group (p<0.03, Figure 2E). For TCDD acute exposure, most ABC genes showed an overexpression among the tested concentrations. ABCB5 (1.0 nM), ABCC1 (0.1 nM) and ABCC4 (1.0 nM) showed significantly increased expression in relation to the control group. It also observed for the ABCB5 and ABCC1 genes in relation to the control group. The ABCB5 gene showed 48% overexpression at the highest concentration of TCDD (1.0 nM) when compared to the control group and the intermediate concentration (p<0.03, Figure 2B). In addition, the gene ABCC1 presented 20% overexpression when compared with the control group and 26% in comparison to the lowest tested concentration (p<0.03, Figure 2C). Lastly, overexpression of 33% was observed for the ABCC4 gene after exposure to the highest TCDD concentration in relation to the control group, as well as the lowest concentration (p<0.02, Figure 2E). Despite that, no differences were found for ABCC2 gene expression (Figure 2D). Differently than observed to acute exposure, after 15 days, there were no significant alterations of gene expression levels for ABCB1, ABCB5, ABCC1, ABCC2, and ABCC4 genes, for either BDE-209 or TCDD exposures.
Figure 1: Cell viability determined by Neutral Red Assay in B16-F1 cells exposed to BDE-209 and TCDD for 24 h and 15 days. Mean (horizontal bar) and independent experiments (dots). Kruskal-Wallis test. Value of p<0.05 was considered as statistically significant; N=3.
Figure 2: Gene expression of A) ABCB1, B) ABCB5, C) ABCC1, D)ABCC2 and E) ABCC4 in B16-F1 cells after 24 h exposure to BDE-209 and TCDD contaminants at 0.01, 0.1 and 1.0 nM. Kruskal-Wallis followed by Dunn's post-test. Value of p<0.05 was considered as statistically significant and p<0.08 to ABCB1 gene. Different letters indicate differences among the three concentrations of the same chemical and asterisk means differences in relation to the control. Mean+SEM; (N=3).
Differently from the results of gene expression, alterations in the ABC proteins activity due to exposure to both tested compounds were found only after chronic exposure. There were no statistically significant changes in fluorescence intensity emitted by Rhodamine B and Calcein AM after 24 h-exposure to BDE-209 and TCDD. Despite that, the values presented a similar pattern among groups from tested POPs concentrations, with a concentration-dependent decrease trend of the activity for BDE-209 (rhodamine B and calcein AM), and an increase trend for TCDD (calcein AM) (Figure 3).
Figure 3: A & B) Gene expression of ABCB1, ABCB5, ABCC1, ABCC2 and ABCC4 in B16-F1 cells after 15-day exposure to BDE-209 and TCDD at 0.01, 0.1 and 1.0 nM. Mean+SEM. Kruskal-Wallis test. Value of p<0.05 was considered as statistically significant; N=3.
Conversely, significant changes in fluorescence intensity were detected after 15-day of exposure to BDE-209 and TCDD. For BDE-209, an increase in rhodamine B fluorescence intensity was observed for the highest concentration (1.0 nM) compared to the lowest and intermediate concentrations (50%), and to the control group (30%, p<0.02, Figure 4A), but when compared to the control group, Dunn's post hoc test did not identify in which pairs of groups the statistical difference was found at the same level of significance. No significant changes were observed for Calcein AM (Figure 4B). For TCDD, the calcein AM fluorescence intensity decreased (33%) in the intermediate (0.1 nM) and the highest (1.0 nM) concentrations in comparison to the control (p<0.02, Figure 4B), but no effects on rhodamine B was observed.
Figure 4: ABC transporters activity after 24 h-exposure to BDE-209 and TCDD relative to vehicle (control). A) Rhodamine B was used to measure MDR (ABCB genes) activity, whereas B) calcein AM was used for MDR (ABCB genes) and MRP-type transporters (ABCC genes) activity. Substrates selectivity based on Legrand et al. (1998) and Mazur et al. (2015). Mean (horizontal bar) and independent experiments (symbols). Kruskal-Wallis test. Value of p<0.05 was considered as statistically significant; N=3.
Discussion
The present study reports the effects of BDE-209 and TCDD exposure in immortalized murine tumor cells (B16-F1) in order to evaluate whether these compounds can modulate cell malignance. This is an innovative approach since environmental contaminants are generally investigated only for their potential carcinogenicity [28]. The results of cell viability confirm the low toxicity of BDE-209 and TCCD to melanoma (B16-F10) cells, but environmental changes can regulate cells’ phenotype as described by Nobili et al. [8]. Also, our recent studies are an example of chemicals roles in phenotype modulation: cylindrospermopsin in HepG2 cells and tribromphenol in B16-F1 cells [26, 29]. The overexpression of ATP-binding cassette transporters genes after acute exposure and the modulation of the ABC transporters activity after chronic exposure to BDE-209 and TCDD, indicates cell modulations that could increase the cancer malignancy and bring negative consequences for the treatment of the disease. The modulation of gene expression by BDE-209 and TCDD after acute exposure is particularly important as the ABC transporters drive the malignancy in a diversity of cancers [30]. BDE-209 has not been correlated with cancer diseases yet, but BDE-209 exposure led to increase of ABCB1 and ABCC4 genes expression, an ABC transporter of MDR and MRP subfamilies, related with malignant phenotype in tumor models [8].
The overexpression of ABCB1 gene at the highest tested concentration of BDE-209 is a relevant data addressing important information as an increase of MDR (Rhodamine B efflux) activity was also observed. The same was reported by Andersen et al. in adenomas and described as a protective factor against further environmentally induced genetic damages [31]. The relevance of regulation of both genes is related to the involvement of ABC transporters and malignancies of cancer, or the further effect in the protein activity at long-term exposure [8]. Foremost, P-glycoprotein and multidrug-associated protein 2 (MRP2) are the major drug efflux transporters associated with multiple drug resistance phenotypes and cancer treatment failure [32]. The upregulation of ABCC4 suggests a close correlation with the increase of protein activity, showing phenotype changes in B16-F1 cells exposed to contaminants at the same concentration range previously encountered in human plasma. According to Nobili et al. the ABCC4 (MRP4) expression is highly associated with clinical sequels in patients with neuroblastoma [8]. Recently, studies have reported the upregulation of ABC transporters genes by pollutants, highlighting how important these proteins are in building resistance toward chemotherapeutics [33-35]. Thus, the current study suggests that BDE-209 could play an important role in cancer disease.
TCCD exposure caused an upregulation in the expression of ABCB5, ABCC1 and ABCC4 genes, leading to a quite different set of discussions. The overexpression of ABCB5 gene was reported to induce dacarbazine, doxorubicin and temozolomide resistance in chemotherapy treatment of melanoma [36]. The current study is aligned with the study of Chartrain and collaborators showing modulation of ABCB5 genes after TCCD exposure. The long-term exposure to TCDD led to decreased ABC transporters activity which is opposite to observed for BDE-209, while the acute exposure triggered changes in the expression of ABCC1 and ABCC4 genes. The ABCC1 gene is related to the efflux of metabolites and its inhibition leads to DNA adducts after benzo[a]pyrene (BaP) exposure. The upregulation of ABCC1 and ABCC4 by high TCDD concentration has already been described in lung bronchoalveolar cells (H358), showing that different types of cancer present distinct results [37]. The overexpression of ABCC4 gene after exposure to both tested compounds also export endogenous substrates, or regulates the growth of lung cancer, lymphoma and is associated with chemotherapy sensitivity [38, 39].
Currently, there are only a few studies correlating PBDEs and gene expression of the ATP-binding cassette system, highlighting the novelty of this study. In human and rodent cells, drug carriers are induced by nuclear receptors as well as MRP2 in primary human hepatocytes [40, 41]. CAR receptor (constitutive androstane receptor) functions as a sensor for endobiotic and xenobiotic substances, and it is also implicated with the expression of MRP2 human hepatocytes [42]. Therefore, these receptors may be the key to understand the mechanisms associated with the regulation of ABC transport system genes in B16-F1 cells. The current data showed that, at first, cells increase gene expression as a defense against the xenobiotics TCDD and BDE-209 but later, gene regulation returns to normal levels. This could be explained by the synthesis of ABC transporters in appropriate quantities to deal with the insult. Finally, at the end of chronic exposure, we still detect increased ABC transporters activity that is explained by the protein long half-life (t1⁄2 = 5 days) [43].
In general, some environmental chemicals and their metabolites interact with ATP-binding transporters, functioning as potential substrates or inhibitors [44, 45]. This is also evident in the activation of PGP (MDR/P-glycoprotein) and BCRP (Breast Cancer Resistant Protein), which are membrane-bound efflux transporters that carry multiple chemical classes. However, little is known about the role of PBDEs in the functioning of ABC carriers [46]. Additionally, the present data bring more information to increase the knowledge correlating ABC transporters proteins, environmental contaminants and cancer disease. The current study clearly shows the ability of environmental contaminants such as BDE-209 and TCDD to modulate the genes regulation of ATP-binding cassette transporters. Finally, BDE-209 and TCDD acted differently in relation to gene expression and protein activity, as BDE-209 had a greater effect upregulating ABCB1 and ABCC4 genes, while TCDD showed greater effects on ABCB5, ABCC1 and ABCC4 genes, but both compounds modulated protein activity at long-term exposure. The differences in gene expression induced by BDE-209 and TCDD in the present study showed the need and relevance to consider mixtures in future toxicological studies.
These findings deserve attention as ABC transporters are responsible for the efflux of xenobiotics, like chemotherapeutic drugs for tumor cells, or in the role of pollutants in cancer susceptibility. We are aware that our study brings more questions than answers, but it opens the opportunity for future investigations about pollutants and cancer disease. We propose that further studies should investigate the ability of these compounds to interfere with the prognosis of cancer.
Acknowledgement
The authors thank Dr. Magda CV Costa Ribeiro and Dr. Silvio S Veiga for all laboratory assistance with the equipment for molecular analysis. This study was supported by The Brazilian National Council for Scientific and Technological Development Agency - CNPq (Finance Code 480707/2013-8) and the Brazilian Coordination for the Improvement of Higher Education Personnel Agency - CAPES. Also, the authors thank very much Dr. Ana Cristina Grodzki for English revision.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 09, Aug 2021Accepted: Mon 23, Aug 2021
Published: Thu 02, Sep 2021
Copyright
© 2023 Ciro A. Oliveira Ribeiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2021.02.01
Author Info
Micheli de Marchi Erick E. Moggio Francisco Filipak Neto Patricia E.M. Brito Benisio F. Silva Filho Ciro A. Oliveira Ribeiro
Corresponding Author
Ciro A. Oliveira RibeiroCellular Toxicology Laboratory, Department of Cellular Biology, Biological Sciences Sector, Polytechnic Center/Federal University of Paraná, Curitiba, Paraná, Brazil
Figures & Tables




References
1. Mânica
A, da Silva Rosa Bonadiman B, Cardoso AM, Paiz A, Siepko C et al. (2019) The signaling effects of ATP on
melanoma-like skin cancer. Cell Signal
59: 122-130. [Crossref]
2.
INSTITUTO NACIONAL DE CÂNCER (2020)
Tipos de câncer: Câncer de pele não melanoma. Rio de Janeiro: INCA.
3. Casalta Lopes J, Abrantes AM, Laranjo
M, Rio J, Gonçalves AC et al. (2011) Efflux Pumps Modulation in Colorectal
Adenocarcinoma Cell Lines: The Role of Nuclear Medicine. J Cancer Ther 2: 408-417.
4. Levine EA, Holzmayer TA, Roninson IB,
Gupta TKD (1993) MDR-1 expression in metastatic malignant melanoma. J Surg
Res 54: 621-624. [Crossref]
5. Crawford RR, Potukuchi PK, Schuetz
EG, Schuetz JD (2018) Beyond Competitive Inhibition: Regulation of ABC
Transporters by Kinases and Protein-Protein Interactions as Potential
Mechanisms of Drug-Drug Interactions. Drug Metab Dispos 46: 567-580. [Crossref]
6.
Locher
KP (2016) Mechanistic diversity in ATP-binding cassette (ABC)
transporters. Nat Struct Mol Biol 23: 487-493. [Crossref]
7.
Chen
KG, Valencia JC, Gillet JP, Hearing VJ, Gottesman MM (2009) Involvement of ABC
transporters in melanogenesis and the development of multidrug resistance of
melanoma. Pigment Cell Melanoma Res 22: 740-749. [Crossref]
8.
Nobili S, Lapucci A, Landini I,
Coronnello M, Roviello G et al. (2020) Role of ATP-binding cassette transporters in cancer
initiation and progression. Semin Cancer Biol 60: 72-95. [Crossref]
9. Houghton AN, Polsky D (2002) Focus on
melanoma. Cancer Cell 2:
275-278. [Crossref]
10.
Yu G, Bu Q, Cao Z, Du X, Xia J et al.
(2016) Brominated flame
retardants (BFRs): A review on environmental contamination in China. Chemosphere
150: 479-490. [Crossref]
11. Carpenter DO (2013) Effects of
Persistent and Bioactive Organic Pollutants on Human Health. Wiley: Hoboken,
New Jersey.
12.
Wang
Y, Chen T, Sun Y, Zhao X, Zheng D et al. (2019) A comparison of the thyroid
disruption induced by decabrominated diphenyl ethers (BDE-209) and
decabromodiphenyl ethane (DBDPE) in rats. Ecotoxicol Environ Saf 174: 224-235. [Crossref]
13.
Stasinska
A, Reid A, Hinwood A, Stevenson G, Callan A et al. (2013) Concentrations of
polybrominated diphenyl ethers (PBDEs) in residential dust samples from Western
Australia. Chemosphere 91:
187-193. [Crossref]
14. Besis A, Samara C (2012)
Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments
-- a review on occurrence and human exposure. Environ Pollut 169: 217-229. [Crossref]
15.
Pesatori
AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA (2009) Cancer incidence in
the population exposed to dioxin after the “Seveso accident”: twenty years of
follow-up. Environ Health 8: 39. [Crossref]
16.
World
Health Organization (2016) Dioxins and
their effects on human health.
17.
Travis
CC, Hattemer Frey HA (1991) Human exposure to dioxin. Sci Total Environ
104: 97-127. [Crossref]
18.
He
Y, Peng L, Zhang W, Liu C, Yang Q et al. (2018) Adipose tissue levels of
polybrominated diphenyl ethers and breast cancer risk in Chinese women: A
case–control study. Environ Res 167:
160-168. [Crossref]
19.
Hoffman
K, Lorenzo A, Butt CM, Hammel SC, Henderson BB et al. (2017) Exposure to flame
retardant chemicals and occurrence and severity of papillary thyroid cancer: A
case-control study. Environ Int 107:
235-242. [Crossref]
20.
Pelcl
T, Skrha Jr J, Prazny M, Vlckova S, Fenclova Z et al. (2018) Diabetes,
Cardiovascular Disorders and 2,3,7,8- Tetrachlorodibenzo-p-Dioxin Body Burden in Czech Patients 50
Years After the Intoxication. Basic Clin Pharmacol Toxicol 123: 356-359. [Crossref]
21.
Brito
PM, Biscaia SMP, de Souza TL, Ramos AB, Leão Buchir J et al. (2020) Oral
exposure to BDE-209 modulates metastatic spread of melanoma in C57BL/6 mice
inoculated with B16eF10 cells. Chemosphere 260: 127556. [Crossref]
22.
Frederiksen
M, Thomsen C, Frøshaug M, Vorkamp K, Thomsen M et al. (2010) Polybrominated
diphenyl ethers in paired samples of maternal and umbilical cord blood plasma
and associations with house dust in a Danish cohort. Int J Hyg Environ
Health 213: 233-242. [Crossref]
23.
Collins
JJ, Budinsky RA, Burns CJ, Lamparski LL, Carson ML et al. (2006) Serum dioxin
levels in former chlorophenol workers. J Exp Sci Environ Epidemiol
16: 76-84. [Crossref]
24.
Leonetti C, Butt CM, Hoffman K, Hammel
SC, Miranda ML et al. (2016)
Brominated flame retardants in placental tissues: associations with infant sex
and thyroid hormone endpoints. Environ Health 15: 113. [Crossref]
25. Repetto
G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell
viability/cytotoxicity. Nature
Protoc 3: 1125-1131. [Crossref]
26.
Liebel S, de Oliveira Rebiero CA, de
Magalhães VF, da Silva RC, Rossi SC et al. (2015) Low concentrations of
cylindrospermopsin induce increases of reactive oxygen species levels,
metabolism and proliferation in human hepatoma cells (HepG2). Toxicol In
Vitro 29: 479-488. [Crossref]
27.
Vernhet
L, Courtois A, Allain N, Payen L, Anger JP et al. (1999) Overexpression of the
multidrug resistance-associated protein (MRP1) in human heavy metal-selected
tumor cells. FEBS Lett 443: 321-325. [Crossref]
28.
Irigaray
P, Newby JA, Clapp R, Hardell L, Howard V et al. (2007) Lifestyle-related
factors and environmental agents causing cancer: an overview. Biomed
Pharmacother 61: 640-658. [Crossref]
29.
de
Souza Salgado YC, Ferreira MB, da Luz JZ, Neto FF, de Oliveira Ribeiro CA
(2018) Tribromophenol affects the metabolism, proliferation, migration and
multidrug resistance transporters activity of murine melanoma cells B16F1. Toxicology
In Vitro 50: 40-46. [Crossref]
30.
Sun
YL, Patel A, Kumar P, Chen ZS (2012) Role of ABC transporters in cancer
chemotherapy. Chin J Cancer 31:
51-57. [Crossref]
31.
Andersen
V, Vogel U, Godiksen S, Frenzel FB, Sæbø M et al. (2013) Low ABCB1 gene expression is an early event in
colorectal carcinogenesis. PLoS One 8: e72119. [Crossref]
32. Lu JF, Pokharel D, Bebawy M (2017) A
novel mechanism governing the transcriptional regulation of ABC transporters in
MDR cancer cells. Drug Deliv Transl Res 7: 276-285. [Crossref]
33.
Franzellitti S, Capolupo M, Wathsala
RHGR, Valbonesi P, Fabbri E (2019) The Multixenobiotic resistance system as a possible
protective response triggered by microplastic ingestion in Mediterranean
mussels (Mytilus galloprovincialis): Larvae and adult stages. Comp Biochem
Physiol C Toxicol Pharmacol 219:
50-58. [Crossref]
34. Han J, Park JC, Kang HM, Byeon E,
Yoon DS (2019) Adverse effects, expression of defense-related genes, and
oxidative stress-induced MAPK pathway in the benzo[α]pyrene-exposed rotifer
Brachionus rotundiformis. Aquat Toxicol 210: 188-195. [Crossref]
35.
Leal Alvarado DA, Estrella Maldonado
H, Sáenz Carbonell L, Ramírez Prado JH, Zapata Pérez O et al. (2018) Genes coding for transporters showed
a rapid and sharp increase in their expression in response to lead, in the
aquatic fern (Salvinia minima Baker). Ecotoxicol Environ Saf 147: 1056-1064. [Crossref]
36.
Chartrain
M, Riond J, Stennevin A, Vandenberghe I, Gomes B et al. (2012) Melanoma
chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One 7: e36762. [Crossref]
37.
Gelhaus
SL, Gilad O, Hwang WT, Penning TM, Blair IA (2012) Multidrug resistance protein
(MRP) 4 attenuates benzo[a]pyrene-mediated DNA-adduct formation in human
bronchoalveolar H358 cells. Toxicol Lett 209: 58-66. [Crossref]
38.
Van
Aubel RAMH, Smeets PHE, van den Heuvel JJMW, Russel FGM (2005) Human organic
anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite
urate with multiple allosteric substrate binding sites. Am J Physiol Renal
Physiol 288: F327-F333. [Crossref]
39.
Zhao
X, Guo Y, Yue W, Zhang L, Gu M et al. (2014) ABCC4 is required for
cell proliferation and tumorigenesis in non-small cell lung cancer. Onco
Targets Ther 7: 343-351. [Crossref]
40.
Staudinger
JL, Madan A, Carol KM, Parkinson A (2003) Regulation of drug transporter gene
expression by nuclear receptors. Drug Metab Dispos 31: 523-527. [Crossref]
41.
Geick
A, Eichelbaum M, Burk O (2001) Nuclear receptor response elements mediate
induction of intestinal MDR1 by rifampin. J Biol Chem 276: 14581-14587. [Crossref]
42.
Kast
HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM et al. (2002) Regulation of
multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors
pregnane X receptor, farnesoid X-activated receptor, and constitutive
androstane receptor. J Biol Chem 277:
2908-2915. [Crossref]
43.
Wakabayashi
Y, Kipp H, Arias IM (2006) Transporters on demand: intracellular reservoirs and
cycling of bile canalicular ABC transporters. J Biol Chem 281: 27669-27673. [Crossref]
44.
Mazur CS, Marchitti SA, Dimova M,
Kenneke JF, Lumen A et al. (2012) Human and rat ABC transporter efflux of bisphenol A and bisphenol A
glucuronide: interspecies comparison and implications for pharmacokinetic
assessment. Toxicol Sci 128:
317-325. [Crossref]
45. Mazur CS, Marchitti SA, Zastre J (2015) P-glycoprotein inhibition by the agricultural pesticide propiconazole and its hydroxylated metabolites: Implications for pesticide-drug interactions. Toxicol Lett 232: 37-45. [Crossref]
46. Marchitti SA, Mazur CS, Dillingham CM, Rawat S, Sharma A et al. (2017) Inhibition of the Human ABC Efflux Transporters P-gp and BCRP by the BDE-209-47 Hydroxylated Metabolite 6-OH-BDE-47: Considerations for Human Exposure. Toxicol Sci 155: 270-282. [Crossref]
