Awake Thoracic Surgery with Extracorporeal Membrane Oxygenation
A B S T R A C T
A 75-year old patient with a right sided secondary pneumothorax and prolonged air leak showed upper lobe predominant bullous emphysema. Due to the patients extremely reduced general condition neither an open approach nor a thoracoscopic approach seemed possible. Hence, we performed an awake lung volume reduction surgery with perioperative single site veno-venous extracorporeal membrane oxygenation. No heparin was administered. The extracorporeal membrane oxygenation (ECMO) could be weaned up to the second postoperative day. The further postoperative course was uneventful. This current case suggests that combining awake surgery with extracorporeal membrane oxygenation could be a future concept in extremely compromised patients.
Keywords
Awake thoracic surgery, thoracoscopic surgery, extracorporeal membrane oxygenation, lung volume reduction surgery
Introduction
The use of awake (AW) thoracic surgery may be beneficial, especially due to the abandonment of muscle relaxants; a faster postoperative recovery and a reduction of stress hormones have been described1. Nevertheless, the impact especially on severely compromised patients remains unclear [1]. We report on an extended indication for AW thoracic surgery to ECMO-augmented-AW thoracic surgery due to severest respiratory compromise of the patient.
Case Report
A 75-old male (176cm/ 60kg) presented with acute dyspnea due to secondary pneumothorax with partially collapsed right lung. Initial treatment was a thoracic drain. The medical history of the patient showed COPD GOLD IV Group D with home oxygen therapy of 3.5 l/min at rest. A prolonged air leak and inability to remove the drain resulted and was caused by severe upper lobe predominant bullous emphysema. Despite initial conservative management, no reduction of the air leak was noticed. Due to this we indicated an operative therapy. On the other hand, due to the patient’s overall condition (O2 requirement by severe emphysema; bedridden due to dyspnea; lung function not feasible) an open thoracic approach was ruled out. A thoracoscopic approach with single lung ventilation or a sole non-intubated approach seemed not possible due to the severe emphysema with need for O2 at rest. Preoperative blood gas values were SO2 88% and pO2 48mmHg.
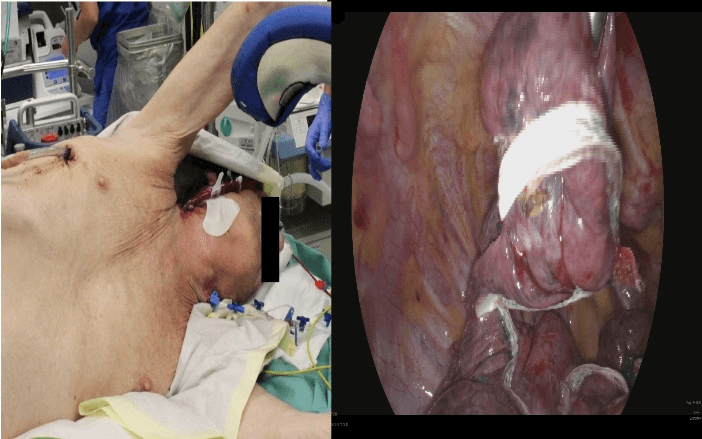
We decided to perform an awake lung volume reduction surgery (LVRS) on the right side with perioperative v-v ECMO support. Under local anesthesia and beginning sedation (intravenous Dexmedetomidine) a 27 Fr double lumen cannula (Maquet) was inserted via the right jugular vein (echocardiographic and fluoroscopic guidance). For analgesia we used intravenous remifentanil and a plexus block. No heparin was administered. Neither an intubation nor noninvasive ventilation was performed. The ECMO (Maquet Cardio Help with an HLS advanced 7.0 Oxygenator) was operated with a blood flow of 3l/min and a FIO2 of 50%. The patient remained cardiopulmonary stable and responsive. The level of sedation was increased, and the patient was placed in a left lateral decubitus position. After collapsing the right lung via a standard three port thoracoscopic approach the patient still remained stable (no catecholaminergic support; respiratory balanced at a respiratory rate of 5/min). A ruptured bulla was found intraoperatively. We proceeded with an uneventful thoracoscopic LVRS on the right upper lobe (Figure 2). An additional talc pleurodesis was performed. Two standard thoracic drainages were placed and put on suction after an operative time of 49 minutes. No relevant air leak was detectable. With wound closure, sedation was already reduced and tapered (Figure 3).
Figure 1: Photography showing the non-intubated patient direct preoperative and the intraoperative thoracoscopic aspect with the mostly resected part of the right upper lobe.
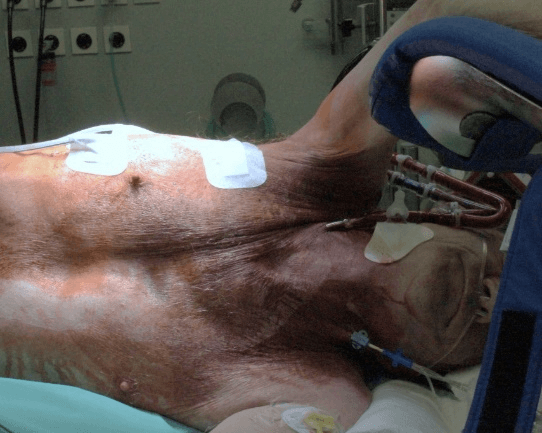
Figure 2: Photography of the non-intubated patient with the v-v-ECMO directly postoperative.
Already during repositioning, the patient was fully awake and without pain. ECMO support was continued unchanged and the patient admitted to the ICU for further monitoring and respiratory exercises. ECMO therapy was weaned and successfully removed on the morning of the second postoperative day. No anticoagulation was administered during ECMO therapy instead only routine coagulation measurements. After removal of the ECMO heparin was administered prophylactically. the patient returned to the normal ward the fourth postoperative day. The postoperative blood gas analysis showed a SO2 97% and pO2 76 mmHg at 4LO2/min. 20 days postoperatively (complication of clostridium difficile infection) the patient was discharged home with the same oxygen therapy as before the event. An interview the 10th day after discharge revealed the patient well and that the whole procedure was not perceived as unpleasant. After 6 month the patient was still in a stable condition like before the pneumothorax.
Comment
It is well known that an intubation in COPD patients leads to further morbidity especially in an exacerbated situation. Postoperative pulmonary complication rates in COPD patients range from 2-19% in non-cardiac surgery whereas for lung resections this rate increases to 4-41% [2, 3]. This relationship led to the concept of an extracorporeal gas exchange to avoid intubation and mechanical ventilation in these patients [4]. AW thoracic surgery may have beneficial effects in an overall population but the impact especially on severely compromised patients remains unclear1. Perioperative ECMO support in thoracic surgery is well established especially for airway management. Nevertheless, rates of up to 20 % for bleeding complications caused by the proposed need for anticoagulation limit its liberal use [5]. Due to positive experience in v-v- ECMO patients without systemic anticoagulation (heparin coated systems in short- to midterm use) we did not use systemic heparinization in this case. Until now, to our knowledge, ECMO was used only in conventional thoracic surgery and not in AW thoracic surgery. In this case we successfully combined these two approaches with the aim of further minimizing deleterious side effects of mechanical ventilation in a severely compromised patient.
Conclusion
Combining AW thoracic surgery and perioperative ECMO is feasible. This may be a future concept to allow operative therapy in respiratory extremely compromised patients in far more cases than the described LVRS. To abandon systemic heparinization might be suggested in similar cases.
Conflicts of Interest
None.
Article Info
Article Type
Case StudyPublication history
Received: Fri 14, Feb 2020Accepted: Fri 06, Mar 2020
Published: Mon 16, Mar 2020
Copyright
© 2023 Vasileios Drosos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.02.15
Author Info
Alexander Kersten Jan Spillner Sebastian Kalverkamp Vasileios Drosos
Corresponding Author
Vasileios DrososDepartment of Thoracic and Cardiovascular Surgery, RWTH Aachen University Hospital, Aachen, Germany
Figures & Tables
References
- Gonzalez Rivas D, Bonome C, Fieira E, Aymerich H, Fernandez R et al. (2016) Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 49: 721-731. [Crossref]
- Licker MJ, Widikker I, Robert J, Frey JG, Spiliopoulos A et al. (2006) Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 81: 1830-1837. [Crossref]
- Numata T, Nakayama K, Fujii S, Yumino Y, Saito N et al. (2018) Risk factors of postoperative pulmonary complications in patients with asthma and COPD. BMC Pulm Med 18: 4. [Crossref]
- Braune SA, Kluge S (2013) Extracorporeal lung support in patients with chronic obstructive pulmonary disease. Minerva Anestesiol 79: 934-943. [Crossref]
- Rinieri P, Peillon C, Bessou JP, Veber B, Falcoz PE et al. (2015) National review of use of extracorporeal membrane oxygenation as respiratory support in thoracic surgery excluding lung transplantation. Eur J Cardiothorac Surg 47: 87-94. [Crossref]
