Application of Cell Culture Models in Studying Viral Diseases (SARS, H1N1 Flu, MERS, COVID-19): A Review
A B S T R A C T
The emergence of recent viral outbreaks, especially the COVID-19 pandemic, and the resulting global mortality and damage has created an urgent need to accelerate the identification, prevention, and treatment of these viral diseases. Due to the limitations in the use of humans, and animal models in terms of time, costs, metabolism differences and ethical issues, in vitro models have become essential in virology research. In the present review, we collected the application of several used cell culture models in studies on four pathogenic viruses - severe acute respiratory syndrome coronavirus (SARS-CoV), influenza A virus (H1N1), middle east respiratory syndrome coronavirus (MERS-CoV), and 2019 novel coronavirus (SARS-CoV-2). These models included, 2D and 3D cell culture (organoids, microfluidic-chips, and bioprinted models). A collection of existing research on these viruses can help fight against the SARS-CoV-2 virus and speed it up against future emerging viruses. Moreover, it can show the shortcomings of in vitro models in virology studies that have been performed to date and provide researchers with new ideas for developing models that are more efficient to deal with similar viral outbreaks.
Keywords
Coronaviruses, influenza A virus, 2D cell culture, organoids, organ-on-chips, 3D bioprinting
Introduction
Over the past two decades, four highly pathogenic and deadly human viruses have emerged. they were severe acute respiratory syndrome coronavirus (SARS-CoV), influenza A virus (H1N1), middle east respiratory syndrome coronavirus (MERS-CoV), and 2019 novel coronavirus (SARS-CoV-2) [1]. Currently, the coronavirus disease-2019 (COVID-19) pandemic caused by SARS-CoV-2 is spreading worldwide and causes deaths and injuries to a large number of people every day due to the lack of knowledge about COVID-19 pathogenesis and the absence of decisive treatment and vaccines available to the public. it is essential to learn more about pathophysiology, in order to prevent further spread and high mortality rate and to identify the best drug targets and vaccines. The use of study models can help to achieve this [2, 3]. Since these four viruses (SARS-CoV, MERS-CoV, H1N1, and SARS-CoV-2) are somewhat similar in some genomic characteristics, pathogenesis, and transmission, the researchers can rely on earlier experience about these viruses to speed up developing new relevant study models [1, 4].
There are generally three types of models for studying viral infections - humans, animals, and cell culture models. Restrictions on animal and human model testing, such as high costs, ethical issues, systemic impact, and animal stress on test results and virus species specificity, have led researchers to prioritize the use of cell culture models. As cell culture models can be designed differently depending on the study’s requirements and the type of virus, they can work with a wide range of host-specific viruses. As a result, these models have various applications in experiments of viral infections, including studying virus pathophysiology, virus isolation, vaccine manufacturing, drug designing, effects of drugs and toxic compounds on cells, and monoclonal antibody production [2, 5]. Models that have been used for SARS-Cov, MERS-CoV, H1N1, and SARS-CoV-2 infections include 2D models (monolayer cultured cell lines), and 3D models (organoid, microfluidic [organ-on-chip], and bioprinted systems) (Figure 1). Earlier findings from cell culture models in similar pathogens (SARS-CoV, MERS-CoV, H1N1) can provide useful ideas to fight against SARS-CoV-2, and future pandemics.
Figure 1: Cell culture models application for viral disease including 2D cell culture, organoid, organ-on-chip, 3D bioprinting.
2D Cell Culture Application for SARS-CoV, MERS-CoV, H1N1 and SARS-CoV-2
The oldest and most common type of cell culture is two-dimensional cell culture [6]. This system is used more than other models (3D models) because it is less costly and often requires fewer facilities, so this method is more accessible (Figure 2). To date, 2D cell cultures have had various applications in virology, so that their results have led to many remarkable advances in cognition, and control of viral diseases such as SARS, MERS, H1N1 and COVID-19 [5]. Therefore, the study of applications of two-dimensional cultures for studies on SARS-CoV-2 and similar viruses can be a great help for further research. One of the most common applications of 2D culture in virology is the effect of various antiviral drugs and compounds. For instance, Martin Spiegel and his teammates indicated that multiplication of SARS-CoV in Vero cell culture is prevented by pretreatment with interferon-beta [7]. Also, Birgit Morgenstern and her co-workers showed that the combination of ribavirin with interferon-beta inhibits SARS-CoV replication in lower concentrations compared to either single treatment [8].
Figure 2: Advantages and disadvantages of 2D cell culture for virology.
In another research, Timothy P. Sheahan and his teammates, revealed that GS-5734 (remdesivir) prevents SARS-CoV and MERS-CoV replication in human airway epithelial cells [9]. Adriaan H. de Wilde and his fellow-worker showed that four compounds - chloroquine, chlorpromazine, loperamide, and lopinavir, inhibit MERS-CoV and SARS-CoV replication in the low micro molar range (50% effective concentrations [EC50s], 3 to 8 M) [10]. Meanwhile, antiviral properties of Chloroquine for SARS-CoV replication in cell culture was reported [11]. In another research, Yuanjiang Zhang demonstrated that siRNA might act as an inhibiting factor of gene expression of SARS-CoV’s Spike protein in 2D cell culture [12]. Other research on the anti-influenza A virus showed three compounds with antiviral activity related to the efficacy of Flos Trollii and antiviral activities of compounds isolated from Pinus densiflora (pine tree) in cell culture [13, 14]. Recent research demonstrated the antiviral activity of ATR-002 and selenium supplementation against influenza A virus in the cell culture [15, 16]. Also, Yejin Jang and his teammates showed that lambda-carrageenan (λ-CGN) could be a promising and preventive agent for several respiratory infections, especially influenza A virus and SARS-CoV-2 [17]. In another research, both pyronaridine and artesunate inhibited the development of SARS-CoV-2 and seasonal influenza A virus in Calu-3 cells. Therefore artesunate and pyronaridine might be effective drugs for COVID-19 or influenza A sufferers [18].
Isolating and identifying viruses in clinical specimens, preparing viruses for vaccines, and studying the viral structure, multiplication cycles, genetics, and virus-host cell interaction are other essential and common applications of 2D cell cultures in virology [5]. Research based on these applications demonstrated that LoVo cells are useful for studying biology and persistent infection of SARS-CoV in in vitro models. They also showed that the expression of angiotensin-converting enzyme 2 (ACE2) is not probably the mere determinant for cells susceptibility to SARS-CoV infection [19]. In addition, Hin Chu and his teammate showed that MERS-CoV actively infected Mo-DCs while SARS-CoV infection was abortive. MERS-CoV induced IFN-γ, IP-10, IL-12, and RANTES in a higher rate than SARS-CoV. MERS-CoV induced more surface expression of MHC II and CD86 than SARS-CoV [20]. In the other research by Isabella Eckerle and her teammate, potential intermediate host species of MERS-CoV were identified, using in vitro testing [21]. Further studies by Kaveh Sadeghi and his colleagues demonstrated that the influenza virus is capable of infecting the pancreas and damaging the pancreas cell line [22].
Recently, with the advent of SARS-CoV-2 and the urgent need to identify the virus for treatment and prevention of the disease, two-dimensional cultures have been used in many studies for various applications like previous similar viral outbreaks. It was shown that the Vero-E6 cell line expresses TMPRSS2 and it is the most widely used cell line to replicate and isolate SARS-CoV-2, compared with other Vero cell clones [23, 24]. Vero-E6 cell line has also been used to evaluate the inhibitory effect of remdesivir and chloroquine and to study morphology and morphogenesis of SARS-CoV-2 in 2D cell culture [25, 26, 27]. Besides, to compare viral tropism, replication and innate immune responses of SARS-CoV-2 with SARS-CoV, MERS-CoV, and the influenza A virus (H1N1), researchers used 2D cell cultures of primary human alveolar epithelial cells and macrophages [28]. In another research, cytopathic effects were observed in human airway epithelial cells after SARS-CoV-2 infection. The isolation of SARS-CoV-2 was demonstrated, and mimic infected human lung cells was made possible in human airway epithelial cells [29]. Another cell line used for research on the SARS-CoV-2 includes LLC-MK2 (Rhesus monkey kidney cells). One study using this cell line showed that aerosol produced by COVID-19 patients could be a source of transmission of the virus [30]. Also, Caco-2 cells (human colon adenocarcinoma), and CL14 detected the sufficiency of SARS-CoV-2 and SARS-CoV replication as shown by cytopathic effects (CPE). Also, it proved that SARS-CoV-2 is more sensitive to TMPRSS2 inhibitors than SARS-CoV and that the anti-SARS-CoV-2 activity of remdesivir and aprotinin can be increased by omeprazole in cell culture [31]. HEK293T (human embryonic kidney grown in tissue culture) can be an alternative cell line for SARS-CoV-2 in vitro infection [32].
3D Cell Culture Application for SARS-CoV, MERS-CoV, H1N1, and SARS-CoV-2
To date, animal and two-dimensional models have been used extensively for virological studies and research. Two-dimensional models have helped us to expand our knowledge of virology. However, they also have limitations and problems, such as not being able to model the inner body due to the growth of cells on smooth layers of glass or plastic, the lack of interaction of cells with each other and their surroundings in two-dimensional culture, not reproducing human disease pathology (Figure 2). Animal models have also restricted uses since they are both time-consuming and costly, not to mention stark differences between human and animal metabolisms. Difficulties mentioned above, led researchers to try other models, especially 3D cell culture models, which are more similar to the human organs [33, 34].
I Organoid
Human organoids, a type of three-dimensional culture, can largely meet the requirements of laboratory models in virological research. Organoids are miniaturized and simplified versions of an organ and are produced in vitro. They can recreate the structure and physiology of human organs to a large extent [35]. Organoids can be suitable models for studying viral diseases, developing antiviral drugs, and so on in virology research. Applying previous research and studies using organoids for viral diseases such as SARS, MERS, H1N1 can give new ideas to study and deal with the current coronavirus (SARS-CoV-2) and similar viruses that may appear in the future. for instance, Jie Zhou and colleagues could show three-dimensional cultured intestinal organoids, also called intestinoids or mini-gut, are very sensitive to MERS-CoV and maintain strong viral replication [36]. Further Kenrie P Y Hui’s research in human airway organoids concluded that human airway organoid provides a related physiological in vitro model. It can be used to examine virus tropism and reproduction ability as well as assessing and comparing different epidemic of animal influenza viruses [37].
Recently, Vanessa Monteil and her teammate showed engineered human kidney and blood vessel organoids can be infected with SARS-CoV-2, and human recombinant soluble ACE2 (hrsACE2) could act as an inhibitor in the early stages of SARS-CoV-2 infections [38]. In another recent research, human bronchial organoids (hBO), which contain basal, club, ciliated, and goblet cells, were generated and used as a model for SARS-CoV-2 research and drug discovery. It was showed that camostat is an inhibitor of TMPRSS2 and can reduce the viral copy number [39]. In addition, human liver ductal organoids and human small intestinal organoids (hSIOs) are sensitive to SARS-CoV-2 and support strong virus replication. Therefore, these can be an in vitro model for SARS-CoV-2 research [40, 41]. In addition, researchers generated a lung organoid model using human pluripotent stem cells (hPSC-LOs) and hPSC-derived colonic organoids (hPSC-COs) that express ACE2 and can support SARS-CoV-2 infection. They found out that FDA-approved drugs such as imagine, mycophenolic acid and quinacrine dihydrochloride can inhibit SARS-CoV-2 entry and decrease the infection of both hPSC-LOs and hPSC-COs with SARS-CoV-2 [42, 43].
Anna Z. Mykytyn and colleagues reported that SARS-CoV-2 is more fusogenic than SARS-CoV, and camostat mesylate is an inhibiting factor for SARS-CoV-2 penetration and multiplication in differentiated organoid-derived human airway cells [44]. Mulay established an alveolar organoid model that enables researchers to evaluate host response to SARS-CoV-2 infection [45]. Moreover, clinical reports of neurological symptoms in COVID-19 patients necessitate research on the effects of SARS-CoV-2 on the central nervous system (CNS). For this purpose, researchers utilized human brain organoids as a useful model to elucidate the susceptibility of the brain to SARS-CoV-2 [46]. Results showed SARS-CoV-2 does not replicate but infect the cortical region of the human brain organoids [47]. In another study, to evaluate virus replication and the host response to infection, Makovoz and colleague infected eye organoids and adult human ocular cells with SARS-CoV-2. Although the degree of replication in the central cornea was not noticeable, the limbus showed the highest susceptibility to infection. In addition, they demonstrated that infections happen directly in the human eye [48].
II Organ-on-Chip
Organ-on-chip, another type of three-dimensional culture, is a microfluidic culture device that involves microchannels. They make it possible to simulate the main parameters (fluid flows, mechanical stimulation, tissue interfaces, etc.) in the physiological environment of tissues and living cells. Microfluidic models can overcome the inefficiencies of the previously mentioned models mostly with spatiotemporal controllability, fluid and gas flow control, physiological limitations of living tissue, and high-output analysis in smaller sample sizes. In recent years, organ-on-chips have been very useful applications in virological research, such as the study of virus-host interactions as well as drug and vaccine responses and development. Moreover, because such research is conducted in micro dimensions and fewer materials and supplies are used, these models could decrease the cost of antiviral drug development (Figure 3) [49-51]. Long Si and colleagues were able to build a human lung airway microfluidic chip that showed the influenza virus replication, host responses to infection, evolution by gene re-assortment or mutation, and effects of antiviral drugs. They successfully demonstrated influenza virus replication, their effect on host cells, and the clinical impact of antiviral drugs. The results showed that this influenza chip may be an alternative preclinical tool for producing antiviral drugs and vaccines [52]. In another recent study, Gard and his teammate designed a human primary airway epithelial cell-based model in a high-output platform. cells cultured at an air-liquid interface (PREDICT96-ALI) in this study could evaluate the therapeutic effect of various small molecules and antiviral agents (such as oseltamivir) against the influenza A and other respiratory viruses, especially coronaviruses [53].
To study recapitulated alveolar-capillary barrier injury and inflammatory responses of SARS-CoV-2 infection, Zhang and his co-worker created a micro-engineered alveolus chip model [54]. Similarly, to recapitulate the intestinal injury and immune response by SARS-CoV-2, Yaqiong Guo and colleagues engineered an intestine-on-chip device [55]. Besides, the microfluidic model of the human blood-brain barrier indicated that spike protein subunits of SARS-CoV-2 can affect the function of blood-brain barrier [56].
Figure 3: Body-on-chip for virology; an ideal multi-organ-on-chip model including SARS-CoV-2 target tissues.
Since ACE2 enzyme, as a specific target for SARS-CoV-2, expressed with different levels in the small intestine, testis, kidneys, heart, thyroid, adipose tissue, lungs, colon, liver, bladder, adrenal gland, blood, spleen, bone marrow, brain, blood vessels and muscle an ideal model should include these tissues interacted each other to mimic the physiological structure of internal organs accurately (Figure 3) [57]. Although the single organ-on-chip models have made great strides in the past decade, they are inadequate models to simulate the complexity, functionality, and integrity of human organs. To solve this problem, the multi-organ-chip models (also known as human-on-a-chip) are options that provides integrated cultured cells of different organs and tissues using microfluidic channels. They can also be a suitable model for virology studies, especially drug development. Therefore, this model can show the effects of viruses, drugs and even vaccines on cells more comprehensively than other models due to the simultaneous study of connective target tissues [6]. Although the multi-organ-chip face many challenges, significant improvements that to date has been made by researchers such as two-organs, three-organs, four-organs, and ten organs on the chip, increase the possibility of creating such a model in the future [34, 51, 58-62].
III 3D Bioprinting
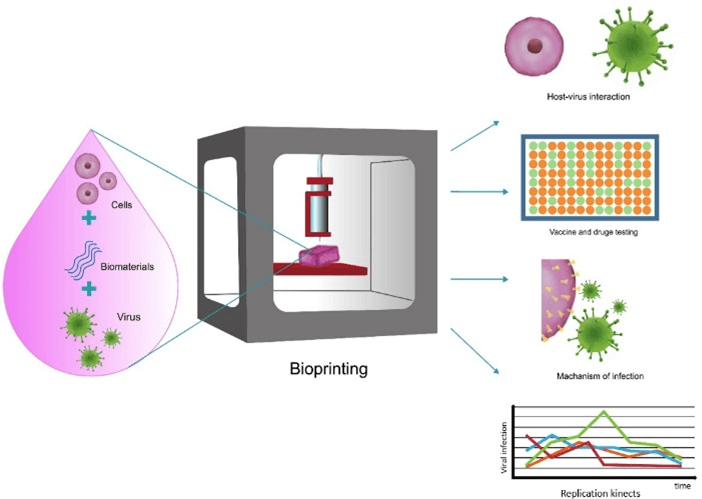
Another 3D cell culture model that has recently drawn more attention in fighting infectious diseases due to its imitation of physiological conditions, complex architectures and structure of biological organs and tissues compared with other models is 3D bioprinting model. This model uses layered cells and biomaterial printing technology to build structures like native tissues using specific tissue cell types in bioink. Several methods are used in biofabrication; the most common of them are extrusion-based, inkjet-based, laser-assisted, stereolithography. However, the extrusion bioprinting method is the most commonly used, because of its convenience and low cost. In virology research, we often require modeling various conditions and certain cell lines that are specific to each type of virus. Due to the selectivity of the underlying factors such as multiple cell types, biomaterials, structure design, and biofabrication methods, bioprinting technology allows researchers to create a wide range of tissues that are accurate in vitro 3D models, based on the type of virus and the required application. As a result, 3D bioprinted structures provide in vitro models of various systems, and make it possible to understand pathogens and pathogen-host interactions, the production of effective vaccines and drug development. Therefore, it can be an ideal model of infectious diseases (Figure 4) [63-65].
Figure 4: Bioprinting for virology application; host-virus interaction, vaccine and drug testing, mechanism of infection, replication kinetic.
Johanna Berg reported 3D bioprinted human models for infection studies on influenza A using a bioink which was made up of 2% alginate and 3% gelatin and fulfilled with 20% Matrigel. She succeeded in providing a 3D lung model with A549 cells by extrusion bioprinting technology. The results suggested that the IL-29 antiviral (interferon λ1) agent shows an immune response in bioprinted cells. The 3D bioprinted model also supported distributed infection with influenza A virus quite similar to the natural human lung [66]. There are various challenges in 3D bioprinting of models regarding the study of viral infections, such as developing suitable biomaterial, cell viability, controlling mechanical stress during bioprinting, ethical issues, and many more. With all those, they have shown to be appropriate in vitro models and have great potential and reliability in the successful construction of tissues, such as liver, kidney, lung, cardiac in various researches [63, 67]. Thus rapid advances in 3D bioprinting technology can develop in vitro platforms which explain the mechanisms of infection, replication kinetics, test vaccines and drugs in inhibiting SARS-CoV-2 infection [64]. Models that have been used for SARS-CoV, MERS-CoV, H1N1, and SARS-CoV-2 infections are summarized in (Table 1).
Table 1: Cell culture models to study
viruses (SARS‐CoV, H1N1, MERS‐CoV, SARS‐CoV‐2).
|
MERS-CoV |
SARS-CoV |
Influenza A (H1N1) |
|||
|
·
Vero cells [32] ·
Vero E6 cells [23,
24, 26, 27, 32] ·
Human airway
epithelial cells [29] ·
human
adenocarcinoma cells (A549), human liver cells (HUH7.0), and human embryonic
kidney cells (HEK-293T) [25] ·
human lung
epithelial (Calu-3) cells [15] ·
primary human
alveolar epithelial cells and macrophages [28] ·
Caco2, CL14,
HT-29, and DLD-1 [31] |
·
Huh7 cells [10] ·
human airway
epithelial cells [66, 68] ·
Monocyte-derived-dendritic
cells (Mo-DCs) [20] ·
human
adenocarcinoma cells (A549), ZN-R, ZLu-R, LGK-1-R, TT-R.B, PO, KN-R, KLu-R [21] |
·
Vero E6 cells [11, 12, 19] ·
Vero cells
caco2, pk-15, cl14 and HPEK [7, 8] ·
human airway
epithelial cells [9, 68] ·
Human embryonic
kidney cell line 293T [63] ·
human embryonic
kidney cells (HEK-293) [69] ·
HeLa, HOS,
C8166, BL41, Hep-2, Huh-7 and NIH3T3 cells [70] ·
LoVo Cells [19] |
·
RAW264.7 cells [13,14] ·
human adenocarcinoma
cells (A549) and Madin-Darby canine kidney (MDCK)cells [16, 17] ·
human lung
epithelial (Calu-3) cells [18] ·
mouse
pancreatic cell line and Mouse Insulinoma cell line
(MIN6) [22] |
2D Cell
Culture |
|
|
·
blood vessel
and kidney organoids [38] ·
bronchial
organoid [39] ·
liver organoid [41] ·
lung organoid [43] ·
small
intestinal organoids [40] ·
colonic
organoid [42] ·
organoid-derived
human airway cells [44] ·
alveolar
organoid [45] ·
brain organoid [47] ·
eye organoid [48] |
·
intestinal
organoid [36] |
- |
·
human airway
organoid [28] ·
intestinal
organoid [71] |
Organoid |
3D Cell
Culture |
|
·
alveolus chip [54] ·
intestine-on-chip
[55] ·
human
blood–brain barrier [72] |
- |
- |
·
human airway
Chip microfluidic culture device [52] ·
airway model
within the PREDICT96-ALI platform [53] |
Microfluidic
device |
|
|
- |
- |
- |
·
3D lung models
with A549 cells [66] |
3D
bioprinting |
|
Conclusion
To fight viral diseases such as COVID-19, it is necessary to design more efficient in vitro models, which produce more reliable results in a shorter time. For this purpose, it is important to study the strengths, weaknesses, and various applications of the in vitro models used in previous research on similar viruses (influenza A, SARS-CoV, MERS-CoV). A collection of the application of these models helps to develop new mixed models for further viral studies. It is also expected that in the future, 3D models especially microfluidics and 3D bioprinting will be more frequently used in virology. In addition, in vitro models will greatly reduce the need for human and animal trials. Thus, we will be prepared for future viral outbreaks.
Author Contributions
Minoo Alavi researched the literature and wrote the article. Afra Hajizadeh and Samira Tajvar discussed, reviewed and edited the manuscript.
Competing Interests
None.
Article Info
Article Type
Review ArticlePublication history
Received: Thu 15, Jul 2021Accepted: Mon 30, Aug 2021
Published: Fri 03, Sep 2021
Copyright
© 2023 Afra Hadjizadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2021.03.07
Author Info
Minoo Alavi Samira Tajvar Afra Hadjizadeh
Corresponding Author
Afra HadjizadehDepartment of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran
Figures & Tables
Table 1: Cell culture models to study
viruses (SARS‐CoV, H1N1, MERS‐CoV, SARS‐CoV‐2).
|
MERS-CoV |
SARS-CoV |
Influenza A (H1N1) |
|||
|
·
Vero cells [32] ·
Vero E6 cells [23,
24, 26, 27, 32] ·
Human airway
epithelial cells [29] ·
human
adenocarcinoma cells (A549), human liver cells (HUH7.0), and human embryonic
kidney cells (HEK-293T) [25] ·
human lung
epithelial (Calu-3) cells [15] ·
primary human
alveolar epithelial cells and macrophages [28] ·
Caco2, CL14,
HT-29, and DLD-1 [31] |
·
Huh7 cells [10] ·
human airway
epithelial cells [66, 68] ·
Monocyte-derived-dendritic
cells (Mo-DCs) [20] ·
human
adenocarcinoma cells (A549), ZN-R, ZLu-R, LGK-1-R, TT-R.B, PO, KN-R, KLu-R [21] |
·
Vero E6 cells [11, 12, 19] ·
Vero cells
caco2, pk-15, cl14 and HPEK [7, 8] ·
human airway
epithelial cells [9, 68] ·
Human embryonic
kidney cell line 293T [63] ·
human embryonic
kidney cells (HEK-293) [69] ·
HeLa, HOS,
C8166, BL41, Hep-2, Huh-7 and NIH3T3 cells [70] ·
LoVo Cells [19] |
·
RAW264.7 cells [13,14] ·
human adenocarcinoma
cells (A549) and Madin-Darby canine kidney (MDCK)cells [16, 17] ·
human lung
epithelial (Calu-3) cells [18] ·
mouse
pancreatic cell line and Mouse Insulinoma cell line
(MIN6) [22] |
2D Cell
Culture |
|
|
·
blood vessel
and kidney organoids [38] ·
bronchial
organoid [39] ·
liver organoid [41] ·
lung organoid [43] ·
small
intestinal organoids [40] ·
colonic
organoid [42] ·
organoid-derived
human airway cells [44] ·
alveolar
organoid [45] ·
brain organoid [47] ·
eye organoid [48] |
·
intestinal
organoid [36] |
- |
·
human airway
organoid [28] ·
intestinal
organoid [71] |
Organoid |
3D Cell
Culture |
|
·
alveolus chip [54] ·
intestine-on-chip
[55] ·
human
blood–brain barrier [72] |
- |
- |
·
human airway
Chip microfluidic culture device [52] ·
airway model
within the PREDICT96-ALI platform [53] |
Microfluidic
device |
|
|
- |
- |
- |
·
3D lung models
with A549 cells [66] |
3D
bioprinting |
|



References
1.
Abdelrahman
Z, Li M, Wang X (2020) Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV,
and Influenza A Respiratory Viruses. Front
Immunology 11: 552909. [Crossref]
2.
Shpichka
A, Bikmulina P, Peshkova M, Kosheleva N, Zurina I (2020) Engineering a Model to
Study Viral Infections: Bioprinting, Microfluidics, and Organoids to Defeat
Coronavirus Disease 2019 (COVID-19). Int
J Bioprint 6: 302. [Crossref]
3.
Takayama
K (2020) In vitro and Animal Models for SARS-CoV-2 research. Trends Pharmacol Sci 41: 513-517. [Crossref]
4.
Mukerjee
N (2020) A Brief Review on the Overview on Immunology of COVID-19: Current
State of the Research. Int J Sci Res 9: 216-222.
5.
Organization
WH (2017) Use of cell culture in virology for developing countries in the
South-East Asia Region.
6.
Shpichka
A, Bikmulina P, Peshkova M, Kosheleva N, Zurina I (2020) Engineering a Model to
Study Viral Infections: Bioprinting, Microfluidics, and Organoids to Defeat
Coronavirus Disease 2019 (COVID-19). Int
J Bioprint 6: 302. [Crossref]
7.
Spiegel
M, Pichlmair A, Mühlberger E, Haller O, Weber F (2004) The antiviral effect of
interferon-beta against SARS-coronavirus is not mediated by MxA protein. J Clin Virol 30: 211-213. [Crossref]
8.
Morgenstern
B, Michaelis M, Baer PC, Doerr HW, Cinatl J Jr (2005) Ribavirin and
interferon-β synergistically inhibit SARS-associated coronavirus replication in
animal and human cell lines. Biochem
Biophys Res Commun 326: 905-908. [Crossref]
9.
Sheahan
TP, Sims AC, Graham RL, Menachery VD, Gralinski LE (2017) Broad-spectrum
antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9: eaal3653. [Crossref]
10.
De
Wilde AH, Jochmans D, Posthuma CC, Zevenhoven Dobbe JC, Van Nieuwkoop S et al.
(2014) Screening of an FDA-approved compound library identifies four
small-molecule inhibitors of Middle East respiratory syndrome coronavirus
replication in cell culture. Antimicrob
Agents Chemother 58: 4875-4884. [Crossref]
11.
Vincent
MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE et al. (2005) Chloroquine
is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2: 69. [Crossref]
12.
Zhang Y, Li T, Fu L, Yu C, Li Y et al. (2004) Silencing SARS-CoV Spike
protein expression in cultured cells by RNA interference. FEBS Lett 560: 141-146. [Crossref]
13.
Ha
TKQ, Lee BW, Nguyen NH, Cho HM, Venkatesan T et al. (2020) Antiviral Activities
of Compounds Isolated from Pinus densiflora (Pine Tree) against the Influenza A
Virus. Biomolecules 10: 711. [Crossref]
14.
Shi
D, Chen M, Liu L, Wang Q, Liu S et al. (2020) Anti-influenza A virus mechanism
of three representative compounds from Flos Trollii via TLRs signaling
pathways. J Ethnopharmacol 253:
112634. [Crossref]
15.
Gong
G, Li Y, He K, Yang Q, Guo M et al. (2020) The inhibition of H1N1 influenza
induced apoptosis by sodium selenite through ROS-mediated signaling pathways. RSC Advances 10: 8002-8007.
16.
Laure
M, Hamza H, Koch Heier J, Quernheim M, Müller C et al. (2020) Antiviral
efficacy against influenza virus and pharmacokinetic analysis of a novel
MEK-inhibitor, ATR-002, in cell culture and in the mouse model. Antiviral Res 178: 104806. [Crossref]
17.
Jang
Y, Shin H, Lee MK, Kwon OS, Shin JS et al. (2020) Antiviral activity of
lambda-carrageenan against influenza viruses in mice and severe acute
respiratory syndrome coronavirus 2 in vitro. bioRxiv.
18.
Bae
JY, Lee GE, Park H, Cho J, Kim YE et al. (2020) Pyronaridine and artesunate are
potential antiviral drugs against COVID-19 and influenza. bioRxiv.
19.
Chan
PK, To KF, Lo AW, Cheung JL, Chu I et al. (2004) Persistent infection of SARS
coronavirus in colonic cells in vitro. J
Med Virol 74: 1-7. [Crossref]
20.
Chu
H, Zhou J, Wong BHY, Li C, Cheng ZS et al. (2014) Productive replication of
Middle East respiratory syndrome coronavirus in monocyte-derived dendritic
cells modulates innate immune response. Virology
454: 197-205. [Crossref]
21.
Eckerle I, Corman VM, Müller MA, Lenk M,
Ulrich RG et al. (2014)
Replicative capacity of MERS coronavirus in livestock cell lines. Emerging Infect Dis 20: 276. [Crossref]
22.
Sadeghi K, Salimi V, Rezaei F, Jalilian
FA, Ghavami N et al. (2020)
Potential of H1N1 influenza A virus as an air borne pathogen to induce
infectivity in pancreas: a mouse model study. J Environ Health Sci Eng 18: 303-310. [Crossref]
23.
Matsuyama
S, Nao N, Shirato K, Kawase M, Saito S et al. (2020) Enhanced isolation of
SARS-CoV-2 by TMPRSS2-expressing cells. Proc
Natl Acad Sci USA 117: 7001-7003. [Crossref]
24.
Zhou
P, Yang XL, Wang XG, Hu B, Zhang L et al. (2020) A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature 579: 270-273. [Crossref]
25.
Harcourt
J, Tamin A, Lu X, Kamili S, Sakthivel SK et al. (2020) Isolation and
characterization of SARS-CoV-2 from the first US COVID-19 patient. bioRxiv. [Crossref]
26.
Vieira DFB, da Silva MAN, Garcia CC,
Miranda MD, Matos AR et al. (2020) Morphology and morphogenesis of SARS-CoV-2 in Vero-E6 cells. Mem
Inst Oswaldo Cruz 116: e200443 [Crossref]
27.
Wang M, Cao R, Zhang L, Yang X, Liu J
et al. (2020) Remdesivir and chloroquine effectively inhibit the recently
emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30: 269-271. [Crossref]
28.
Hui
KPY, Cheung MC, Perera RAPM, Ng KC, Bui CHT et al. (2020) Tropism of the novel
coronavirus SARS-CoV-2 in human respiratory tract: an analysis in ex vivo and
in vitro cultures. Lancet Respir Med 8: 687-695 [Crossref]
29.
Zhu
N, Zhang D, Wang W, Li X, Yang B et al. (2020) A novel coronavirus from
patients with pneumonia in China, 2019. N
Engl J Med 382: 727-733. [Crossref]
30.
Lednicky
JA, Lauzardo M, Fan ZH, Jutla A, Tilly TB et al. (2020) Viable SARS-CoV-2 in
the air of a hospital room with COVID-19 patients. Int J Infect Dis 100: 476-482. [Crossref]
31.
Bojkova
D, McGreig JE, McLaughlin KM, Masterson SG, Widera M et al. (2020) SARS-CoV-2
and SARS-CoV differ in their cell tropism and drug sensitivity profiles. bioRxiv.
32.
Kim
JM, Chung YS, Jo HJ, Lee NJ, Kim MS et al. (2020) Identification of Coronavirus
Isolated from a Patient in Korea with COVID-19. Osong Public Health Res Perspect 11: 3-7. [Crossref]
33.
Antoni
D, Burckel H, Josset E, Noel G (2015) Three-dimensional cell culture: a
breakthrough in vivo. Int J Mol Sci
16: 5517-5527. [Crossref]
34.
Esch
MB, Mahler GJ (2019) Microfluidic Cell
Culture Systems 323-350.
35.
Kim
J, Koo BK, Knoblich JA (2020) Human organoids: model systems for human biology
and medicine. Nat Rev Mol Cell Biol
21: 571-584. [Crossref]
36.
Zhou
J, Li C, Zhao G, Chu H, Wang D et al. (2017) Human intestinal tract serves as
an alternative infection route for Middle East respiratory syndrome
coronavirus. Sci Adv 3: eaao4966. [Crossref]
37.
Hui
KPY, Ching RHH, Chan SKH, Nicholls JM, Sachs N et al. (2018) Tropism,
replication competence, and innate immune responses of influenza virus: an
analysis of human airway organoids and ex-vivo bronchus cultures. Lancet
Respir Med 6: 846-854. [Crossref]
38.
Monteil
V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA et al. (2020) Inhibition of
SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble
human ACE2. Cell 181: 905-913. [Crossref]
39.
Suzuki
T, Itoh Y, Sakai Y, Saito A, Okuzaki D et al. (2020) Generation of human
bronchial organoids for SARS-CoV-2 research. bioRxiv.
40.
Lamers
MM, Beumer J, van der Vaart J, Knoops K, Puschhof J et al. (2020) SARS-CoV-2
productively infects human gut enterocytes. Science
369: 50-54. [Crossref]
41.
Zhao
B, Ni C, Gao R, Wang Y, Yang L et al. (2020) Recapitulation of SARS-CoV-2
infection and cholangiocyte damage with human liver organoids. biorxiv.
42.
Han
Y, Duan X, Yang L, Nilsson Payant BE, Wang P et al. (2021) Identification of
SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589: 270-275. [Crossref]
43.
Han
Y, Yang L, Duan X, Duan F, Nilsson Payant BE et al. (2020) Identification of
Candidate COVID-19 Therapeutics using hPSC-derived Lung Organoids. bioRxiv. [Crossref]
44.
Mykytyn
AZ, Breugem TI, Riesebosch S, Schipper D, van den Doel PB et al. (2020) The
SARS-CoV-2 multibasic cleavage site facilitates early serine protease-mediated
entry into organoid-derived human airway cells. bioRxiv.
45.
Mulay
A, Konda B, Garcia G, Yao C, Beil S et al. (2020) SARS-CoV-2 infection of
primary human lung epithelium for COVID-19 modeling and drug discovery. bioRxiv. [Crossref]
46.
Jacob
F, Pather SR, Huang WK, Wong SZH, Zhou H et al. (2020) Human Pluripotent Stem
Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism. bioRxiv. [Crossref]
47.
Ramani A, Müller L, Ostermann PN,
Gabriel E, Abida Islam P et al. (2020) SARS-CoV-2 targets cortical neurons of 3D human brain
organoids and shows neurodegeneration-like effects. bioRxiv.
48.
Makovoz
B, Moeller R, Eriksen AZ, tenOever BR, Blenkinsop TA (2020) SARS-CoV-2
Infection of Ocular Cells from Human Adult Donor Eyes and hESC-Derived Eye
Organoids. SSRN 3650574. [Crossref]
49.
Bahrami
S, Baheiraei N, Najafi Ashtiani M, Nour S, Razavi M (2020) in Biomedical Applications of Microfluidic
Devices 209-233.
50.
Tang
H, Abouleila Y, Si L, Ortega Prieto AM, Mummery CL et al. (2020) Human
organs-on-chips for virology. Trends
Microbiol 28: 934-946. [Crossref]
51.
Wu
Q, Liu J, Wang X, Feng L, Wu J et al. (2020) Organ-on-a-chip: recent
breakthroughs and future prospects. Biomed
Eng Online 19: 9. [Crossref]
52.
Si L, Prantil Baun R, Benam KH, Bai H,
Rodas M et al. (2019)
Discovery of influenza drug resistance mutations and host therapeutic targets
using a human airway chip. bioRxiv
685552.
53.
Gard
AL, Maloney R, Cain BP, Miller CR, Luu RJ et al. (2020) High-Throughput Human
Primary Cell-Based Airway Model for Evaluating Influenza, Coronavirus, or other
Respiratory Viruses in vitro. bioRxiv.
54.
Zhang
M, Wang P, Luo R, Wang Y, Li Z et al. (2020) A human disease model of
SARS-CoV-2-induced lung injury and immune responses with a microengineered
organ chip. biorxiv.
55.
Guo
y, Luo R, Wang Y, Deng P, Zhang M et al. (2020) Modeling SARS-CoV-2 infection
in vitro with a human intestine-on-chip device. bioRxiv.
56.
Buzhdygan
TP, DeOre BJ, Baldwin Leclair A, Bullock TA, McGary HM et al. (2020) The
SARS-CoV-2 spike protein alters barrier function in 2D static and 3D
microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis 146: 105131. [Crossref]
57.
Li
MY, Li L, Zhang Y, Wang XS (2020) Expression of the SARS-CoV-2 cell receptor
gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9: 45. [Crossref]
58.
Edington
CD, Chen WLK, Geishecker E, Kassis T, Soenksen LR et al. (2018) Interconnected
Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Sci Rep 8: 4530. [Crossref]
59.
Oleaga
C, Bernabini C, Smith AST, Srinivasan B, Jackson M et al. (2016) Multi-organ
toxicity demonstration in a functional human in vitro system composed of four
organs. Sci Rep 6: 20030. [Crossref]
60.
Skardal
A, Murphy SV, Devarasetty M, Mead I, Kang HW et al. (2017) Multi-tissue
interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7: 8837. [Crossref]
61.
Tsamandouras
N, Chen WLK, Edington CD, Stokes CL, Griffith LG et al. (2017) Integrated gut
and liver microphysiological systems for quantitative in vitro pharmacokinetic
studies. AAPS J 19: 1499-1512. [Crossref]
62.
Yang
JW, Shen YC, Lin KC, Cheng SJ, Chen SL et al. (2020) Organ-on-a-Chip:
Opportunities for Assessing the Toxicity of Particulate Matter. Front Bioeng Biotechnol 8: 519. [Crossref]
63.
Chakraborty
J, Banerjee I, Vaishya R, Ghosh S (2020) Bioengineered in Vitro Tissue Models
to Study SARS-CoV-2 Pathogenesis and Therapeutic Validation. ACS Biomater Sci Eng 6: 6540-6555. [Crossref]
64.
de
Melo BA, Benincasa JC, Cruz EM, Maricato JT, Porcionatto MA (2020) 3D culture
models to study SARS-COV-2 infectivity and antiviral candidates: from spheroids
to bioprinting. Biomed J 44: 31-42. [Crossref]
65.
Zimmerling
A, Chen X (2020) Bioprinting for combating infectious diseases. Bioprinting 20: e00104. [Crossref]
66.
Berg
J, Hiller T, Kissner MS, Qazi TH, Duda GN et al. (2018) Optimization of
cell-laden bioinks for 3D bioprinting and efficient infection with influenza A
virus. Sci Rep 8: 13877. [Crossref]
67.
Chameettachal
S, Yeleswarapu S, Sasikumar S, Shukla P, Hibare P et al. (2019) 3D Bioprinting:
Recent Trends and Challenges. J Indian
Institute Sci 1-29.
68.
Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan
TP, et al. (2018) Coronavirus susceptibility to the antiviral remdesivir
(GS-5734) is mediated by the viral polymerase and the proofreading
exoribonuclease. mBio 9: e00221-e00318. [Crossref]
69.
Zhong F, Zhong Z-Y, Liang S, Li X-J (2006) High
expression level of soluble SARS spike protein mediated by adenovirus in HEK293
cells. World J Gastroenterol 12: 1452-1457. [Crossref]
70.
Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M et al.
(2004)
Susceptibility to SARS coronavirus S protein-driven infection correlates with
expression of angiotensin converting enzyme 2 and infection can be blocked by
soluble receptor. Biochem Biophys Res Commun 319: 1216-1221. [Crossref]
71. Huang L, Hou Q, Ye L, Yang Q, Yu Q (2017) Crosstalk between H9N2 avian influenza virus and crypt-derived intestinal organoids. Vet Res 48: 71. [Crossref]
72. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, et al. (2020) The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol Dis 146: 105131. [Crossref]
