Angiosarcoma Secondary to a Paget’s Disease: A Rare Clinical Case With 5 Years of Follow-Up and Review of the Literature
A B S T R A C T
Paget's disease is characterized by disorganized bony tissue due to altered equilibrium of bone formation and resorption. Its malignant transformation is well known and is usually described to an osteosarcoma, often with bad prognosis. In this paper, we present a case clinic of an angiosarcoma from a Paget’s disease of a pelvic bone. The patient underwent a surgical procedure of tumor resection with wide margins (Enneking zone 2-3). The follow-up of 5 years in this case is unique in the literature.
Keywords
Paget’s disease, Angiosarcoma, Pelvic resection, Paget’s sarcomatous transformation
Introduction
Paget's disease was first described by Sir James Paget in 1877 [1]. This disorder is characterized by disorganized bony tissue due to altered equilibrium of bone formation and resorption. Bony tissue becomes structurally weaker, and it can be subject to deformities and pathologic fractures [2, 3]. Malignant transformation of Paget bone disease can occur in approximately 1% of the patients, usually in osteosarcoma, with a very poor prognosis. There are only two cases of bone angiosarcoma in pagetic bones reported in literature [4, 5]. There is a lack of evidence in the current literature about the treatment of these conditions. In this case report, we described an additional case because of its promising outcome at 5 years follow-up despite the high mortality of this disease.
Case Report
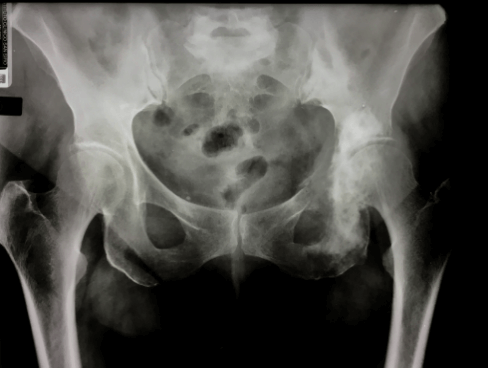
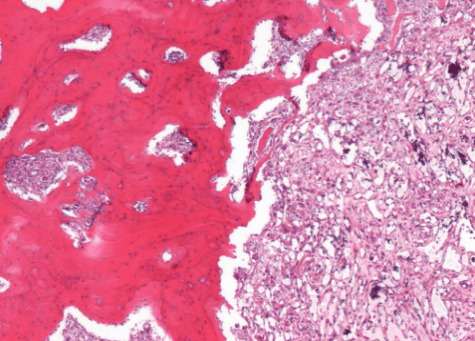
An 80-year-old woman was admitted to the Oncologic Orthopedic Unit of G. Pini Hospital-Milan in March 2005 with lumbo-sciatic pain. X-ray of the pelvis showed anomalies of the bone structure in the area 2-3 of the left hemipelvis (Figure 1). She was known to be affected by Paget disease since the age of 77; in her history, there was an ileo-pubic fracture after trauma at the age of 75. She was under treatment for diabetes and hypertension. She referred to have suffered from left coxalgia associated with pain irradiation to the left limb for almost one year before accessing the ER of our hospital. An open biopsy was performed, and the histological findings were suggestive for malignant mesenchymal pleomorphic and epithelioid neoplasm with morphologic and immune-phenotypical vascular differentiation, consistent with angiosarcoma in Paget disease (Figure 2).
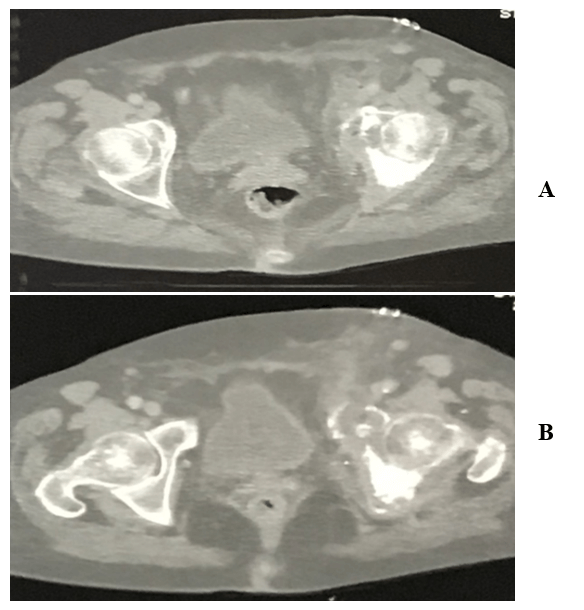
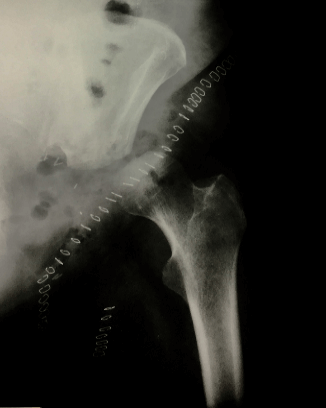
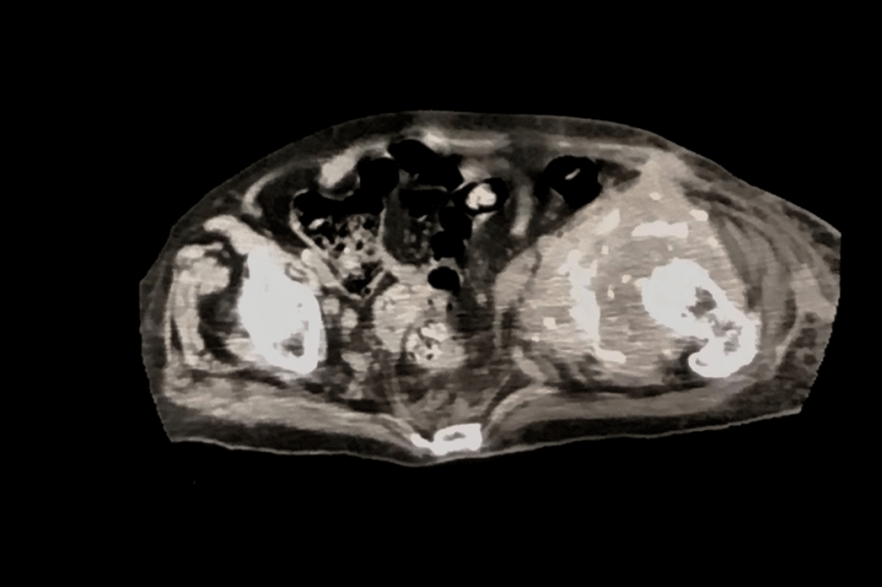
CT-scan showed sarcomatous degeneration with pathological lysis of supra-acetabular area, anterior and posterior cup wall, and ileo- and ischiopubic rami (Figures 3A & 3B). Neoplastic soft tissues infiltrated internal obturator muscle, affecting the coxo-femoral joint, down to obturator foramen. Neoplastic extra compartmental soft tissues spread near femoral vessels, in the area where they emerge from the inguinal canal, with lymph nodes involvement. A skip metastasis was also mentioned in the posterior area of iliac wing, at the edge of the great ischiatic foramen. No secondary neoplastic localizations were found during staging. The surgical treatment proposed for this patient was a wide pelvic resection, including "area 2-3" by Enneking’s classification, without reconstruction (free hip technique) using ileo-inguinal surgical exposure from anterior to posterior in the right semi-lateral recumbent position (Figure 4).
Figure 1: X-rays of the pelvis show anomalies of the bone structure on the area 2-3 of the left hemipelvis.
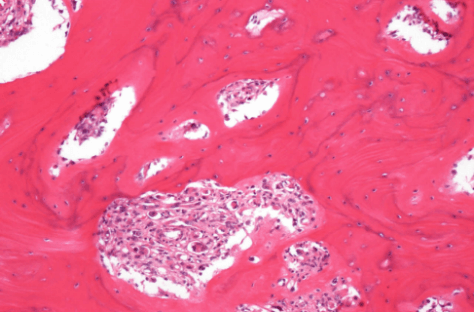
Figure 2: Thick lamellar bone with irregular cement lines infiltrated by atypical blood-vessel forming tumor.
Figure 3: (A-B) CT-scan axial views show pathological lysis of supra-acetabular area, anterior and posterior cup wall, and ileo- and ischiopubic rami.
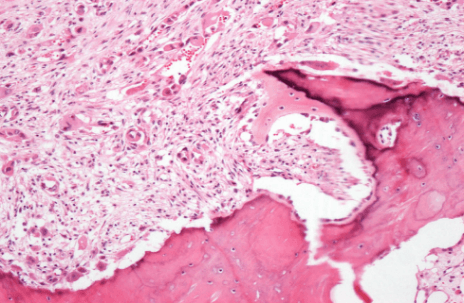
The resected tissue was sent to the Pathology Department for final histological examination which confirmed the bioptic diagnosis of angiosarcoma in Paget’s disease with resection margins that were free of disease. At the gross examination, the hemipelvis resection was infiltrated by a tumor mass of 9, 5 cm of diameter, composed of white-brownish tissue, in part elastic in part calcified, with hemorrhagic areas, that extended up to the acetabular roof. Microscopic examination of the specimen revealed a blood-vessel forming tumor that presented atypical elements and epithelioid aspect, with morphology and immunophenotype consistent with angiosarcoma. The bone infiltrated by the tumor showed features typical of Paget disease of bone: thick trabeculae, disorganization of lamellar bone with a focal mosaic pattern, prominent irregular cement lines focal osteoclastic activity and diffuse fibrosis of marrow (Figures 5 & 6).
Figure 4: Resection of area 2-3 by Enneking’s classification, without reconstruction (free hip technique).
Figure 5: Thick trabeculae, fibrosis, and focal epithelioid features.
Figure 6: Area of diffuse fibrosis and bone necrosis, with epithelioid neoplastic cells focally forming blood-vessel structures.
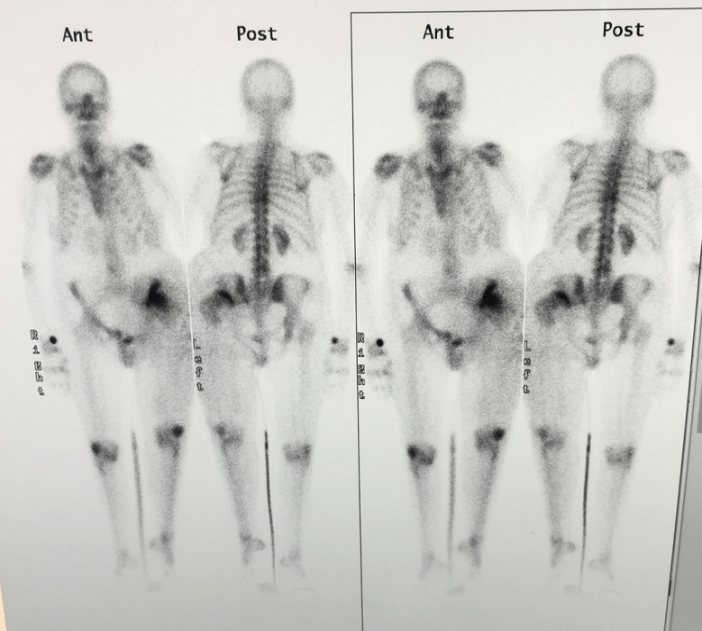
The postoperative course was complicated by surgical wound dehiscence with purulent drainage. Empiric antibiotic therapy was performed with local infection resolution. The patient was re-admitted to the hospital in February 2006 for the onset of swelling in the same area of the surgical treatment, since it was suspicious for malignant recurrence. New CT-scan and bone-scan were then performed: inguinal lesion with increased tracer uptake in the surgical area (Figures 7 & 8). A new FNB (fine needle biopsy) was conducted with a negative result for both recurrence and infection. She continued her follow-up at our institute till the last visit recorded in March 2009, with no evidence of disease. The patient's relatives were telephonically reached some years later, and we were informed that she had passed away due to immobilization syndrome complications in May 2010 at the age of 85.
Figure 7: CT-scan axial plan: Structural alteration of bone and soft tissue in the surgical field.
Figure 8: Bone-scan showing an increased tracer uptake in the surgical area.
Discussion
The incidence of sarcomatous progression in Paget disease of bone (PDB) has been reported by approximately 1%. The malignant transformation is usually to Osteosarcoma (22-90%), but any variant of sarcoma transformation is ideally possible: Fibrous Histiocytoma (26%), Fibrosarcoma (3-25%), Chondrosarcoma (1-15%) [6]. To our knowledge, only two cases of transformation to Angiosarcoma have been described in the literature.
Primitive Angiosarcomas are rare tumors originating from vascular and lymphatic vessels endothelium. They represent just 2% of all soft tissue tumors. The percentage of Angiosarcomas involving bone tissue is 3,6% [7]. Soft tissue sarcomas overall survival is 50-60% at 5 years, whereas angiosarcomas' is 35% [8-11]. Mankin et al. describe in their review a survival rate of 14% at 2,5-years follow-up in Paget Sarcomas [12]. One of the causes of the low survival rate for this disease lies in the late diagnosis since Paget disease is often asymptomatic, and it shows a metastatic pattern from the beginning. Pain onset or worsening and swelling can be related to a malignant progression. The most common sites of sarcomatous transformation are in descending order femur, pelvis and humerus [13].
Early diagnosis is mandatory. The conventional radiologic exam is still the first-line for imaging: cortical bone erosion with lytic bony appearance is the most common X-rays finding. TC-scan and MRI are second-line imaging exams, with whom it is possible to assess the exact disease extent both in bone and soft tissues and its relationship with surrounding nervous and vascular structures, essential to plan the best surgical approach and technique for each case. Bone-scan is usually performed in addition to the previous imaging examinations, and it shows an increase of tracer intake in pathological pagetic bone sections. Yeh et al. described, though, a decrease of tracer intake when sarcomatous progression occurs [14]. Nevertheless, a clear diagnosis can be achieved only with a biopsy.
In our case, the patient was diagnosed with Paget disease at age 77, three years before sarcomatous progression was detected, in line with the diagnosis delay highlighted in the literature. Our patient’s main symptom was pain, without any swelling. According to radiographic images, it was an osteosclerotic lesion with ileo-pubic pathologic fracture. Boulanger et al. described the case of a 61-year-old patient with a 10 cm-diameter swelling at the right hip, corresponding to radiological findings of iliac wing lytic lesion, without joint involvement [5]. The surgical treatment of choice was an en bloc resection of the iliac wing with proximal femur allograft reconstruction.
Chen described a case of an 87-year-old man who underwent shoulder disarticulation due to angiosarcomatous progression in proximal humerus with Paget disease [4]. In our case, the treatment of choice was an en bloc resection of area 2 and area 3 pelvic zone without any reconstruction (free hip technique). In both Boulanger’s and our cases, resection margins were free from tumor cells. No adjuvant therapy was performed in our patient, in contrast to Boulanger’s, because of the different age of the two patients. Our patient died at age 85 from immobilization syndrome complications.
The case we reported is, therefore, a unique finding in the literature of 5-year disease-free survival, in contrast with the data reported by Shaylor et al., according to which no patient had survived at a 5-year follow-up [15].
Conclusion
Sarcomatous transformation in Paget disease is an extremely rare event, and the main histological variant is osteosarcoma. The case here reported is the third documented case of angiosarcoma in Paget present in current literature. The prognosis in sarcomatous Paget variants is usually unfortunate, as primitive angiosarcoma is. Our case-report shows encouraging data for what concerns disease-free survival of sarcomatous progression in Paget disease, and in our opinion, improvement of surgical techniques in association with early diagnosis is mandatory to achieve a better outcome.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 11, May 2020Accepted: Wed 27, May 2020
Published: Thu 04, Jun 2020
Copyright
© 2023 M. Silvia Spinelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CRSS.2020.02.01
Author Info
Andrea Di Bernardo Fabio Sciancalepore Fabio L. Giardina M. Silvia Spinelli Matteo G. Lo Duca Primo A. Daolio Stefano Bastoni
Corresponding Author
M. Silvia SpinelliOrthopaedic Oncology Unit, ASST “Gaetano Pini” - CTO, Milan, Italy
Figures & Tables
References
- J Paget (1877) On a form of chronic inflammation of bones (osteitis deformans). Med Chir Trans 60: 37-64. [Crossref]
- F S Kaplan, F R Singer (1993) Paget’s disease of bone: pathophysiology and diagnosis. Instr Course Lect 42: 417-424. [Crossref]
- R L Merkow, J M Lane (1990) Paget’s Disease of Bone. Orthop Clin North Am 21:171-189. [Crossref]
- K T Chen (1985) Hemangiosarcoma complicating Paget's disease of bone. J Surg Oncol 28: 187-189. [Crossref]
- V Boulanger, D Chauveaux, G Kantor, M J Loyer-Lecestre, J Rivel et al. (1998) Primary angiosarcoma of bone in Paget’s disease. Eur J Surg Oncol 24: 611-613. [Crossref]
- A Hadjipavlou, P Lander, H Srolovitz, I P Enker (1992) Malignant transformation in Paget's disease of bone. Cancer 70: 2802-2808. [Crossref]
- Robin J Young, Nicola J Brown, Malcolm W Reed, David Hughes, Penella J Woll (2010) Angiosarcoma. Lancet Oncol 11: 983-991. [Crossref]
- Simone Mocellin, Carlo R Rossi, Alba Brandes, Donato Nitti (2006) Adult soft tissue sarcomas: conventional therapies and molecularly targeted approaches. Cancer Treat Rev 32: 9-27. [Crossref]
- Matthew G Fury, Cristina R Antonescu, Kimberly J Van Zee, Murray F Brennan, Robert G Maki (2005) A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 11: 241-247. [Crossref]
- R J Mark, J C Poen, L M Tran, Y S Fu, G F Juillard (1996) Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 77: 2400-2406. [Crossref]
- J Fayette, E Martin, S Piperno-Neumann, A Le Cesne, C Robert et al. (2007) Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 18: 2030-2036. [Crossref]
- Henry J Mankin, Francis J Hornicek (2005) Paget’s sarcoma: a historical and outcome review. Clin Orthop Relat Res 438: 97-102. [Crossref]
- C López, D V Thomas, A M Davies (2003) Neoplastic transformation and tumor-like lesions in Paget's disease of bone: a pictorial review. Eur Radiol 13: L151-L163. [Crossref]
- S D Yeh, G Rosen, R S Benua (1982) Gallium scans in Paget’s sarcoma. Clin Nucl Med 7: 546-552. [Crossref]
- Phillip J Shaylor, David Peake, Robert J Grimer, Simon R Carter, Roger M Tillman et al. (1999) Paget’s osteosarcoma: no cure in sight. Sarcoma 3: 191-192. [Crossref]
