Journals
An in vitro feasibility investigation considering primary human melanocytes for spray- grafting of freshly isolated autologous skin cells for burn treatment and a clinical case report
A B S T R A C T
A skin cell-spray grafting technique that enables the on-site application of freshly isolated autologous single cell suspensions was already applied in many cases on caucasian patients with low skin coloration. Our project hypothesis is that these suspensions contain keratinocytes and vital melanocytes, that are of particular interest for the treatment of patients of darker skin color. To test this, we applied an in vitro model, wherein the feasibility of i) isolating and ii) spraying of freshly isolated autologous melanocyte-keratinocyte cell suspensions was investigated. Primary human epidermal keratinocytes (HEKs) and melanocytes (MCs) were isolated from skin biopsies (n=8). Biochemical parameter, cell counts, cell morphology, growth behavior and immunofluorescence results were compared in two groups using MC cultures and co-cultures of MCs with HEKs. Case information on using the method clinically with one patient is included. The sprayed mixed cell suspensions proliferated in all groups without measurable loss of viability, and MCs exhibited a regular cell morphology in monoculture up to passage 4°. The sprayed MCs and HEKs demonstrated in vitro glucose and lactate metabolism that was comparable to the pipetted controls. In co-culture, well distributed CK14+ HEKs and NKI/beteb+ MCs could be demonstrated, which interacted in the in vitro model. The ratio of HEKs : MCs in our primary cultures were microscopically counted (n=8 each) as mean +/- SD 1,211,000 (+/- 574,343) HEK : 99,625 (+/- 59,025) MC; i.e., a ratio of approx. 12 : 1. Using the isolation method clinically for a patient with dark skin coloration after suffering severe second-degree burns shows a satisfying re-pigmentation of the resulting wound post healing. Freshly isolated spray-on melanocyte/keratinocyte suspensions provide for a considerable amount of viable HEKs and MCs. Using MCs in spray-grafting suspensions could represent a promising approach for treating severe partial-thickness burns and innovative therapy developments that also aim to address cosmetic aspects.
Keywords
Autologous skin cells, cell spray grafting, melanocyte-keratinocyte isolation, burns, melanocyte characterization
Introduction
The amount, type, and distribution of the dark biopolymer melanin in the skin contribute to the uniqueness of every person [1, 2]. Already after deep-partial thickness-burn trauma, therapy results can include post-burn hypopigmentation (leucoderma) [3-5]. Visual impressions of body form and skin color are important factors for interactions among humans, especially for facial appearance. Thus, the cosmetic outcome of grafting can be important for an individual’s psychosocial well-being post therapy. Although autologous skin spray grafting [Allouni et al.] was already introduced to complement conventional split-skin grafting in deep partial thickness burns, aspects of re-pigmentation were not studied yet [6-11]. Therapeutically applied melanocytes (MCs) bear the potential for improving cosmetic results due to their re-pigmentation properties but using standard cell isolation techniques that focus on human epidermal keratinocytes (HEKs), they may not have been provided in sufficient amounts [12, 13]. For cell spray grafting approaches, MCs would be ideally isolated in natural ratios compared to HEKs and be kept vital and proliferative post spraying [6].
Using a previously clinically applied cell isolation method, we have demonstrated that autologous freshly isolated HEK containing skin cell suspensions can be used for immediate spray-grafting with clinically satisfying results [7, 8, 14, 15]. This method differs from the process published by of Wood et al. in performing an initial step of separation of the dermal and epidermal layer using the enzyme dispase followed by an extra cell washing step [12]. Thereby, dispase enables to expose the progenitor cell-containing basal layer before the exposure with trypsin for cell liberation, and thus, complete isolation from all relevant skin layers. Our project hypothesis is that the cell suspension isolated with this method contains keratinocytes and vital melanocytes, of particular interest in the treatment of patients with darker skin coloration. We further hypothesized that direct spray deposition after cell isolation would have no negative impact on the cell attachment, survival, and expansion in vitro.
To test this, we applied an in vitro model where we wanted to confirm:
i) a transferability of viable patient’s HEKs and MCs when using developed isolation and spray grafting technique,
ii) the viability of MCs post-spraying, and
iii) the presence of surviving HEKs / MCs sprayed onto an in vitro wound model test surface in native ratios.
Our in vitro results are supported by preliminary data of one clinical case report.
Materials and Methods
Unless otherwise indicated, Biochrom AG (Berlin, Germany) supplied all materials. All media were supplemented with 120 µg/ml Penicillin/Streptomycin (Gibco, Thermo Scientific, Waltham, MA) and 2,5 µg/ml Amphotericin B (Gibco). Pipetted control cultures and sprayed cultures were incubated at 37°C in a humidified atmosphere with 5% CO2 in an incubator (Heraeus BB 6060, Kendro, Langenselbold, Germany).
Skin biopsies – keratinocyte and melanocyte isolation
The ethical committee of the Medical Faculty Charité Campus Virchow-Clinic Berlin, Germany, approved the use of cells for all described in vitro studies. The study used human-derived skin cells and was conducted according to the Declaration of Helsinki principles. Participants provided their written informed consent. Skin biopsies of 11 healthy volunteers undergoing cosmetic surgery were used, which resulted in n=8 experimental setups. Said biopsies were about 2 x 2 cm and up to 6 x 4 cm in size. Our laboratory previously described skin cell isolation for primary HEKs along with cell culture, and we followed the respective experimental protocols [16, 17]. However, for this study, HEKs and MCs were unhinged from the epidermal layer by using 0,05% Trypsin and 0,02% EDTA (Gibco) solution for 15 min at 37°C. After stopping the reaction with PBS containing 10% FCS, cells were centrifuged at 1000 rpm (216 g) for 5 min (Multifuge 3 S-R, Kendro Laboratory Products GmbH, Langenselbold, Germany). The pellet received was suspended in melanocyte specific medium M254 (Cascade Biologics, Inc., Portland, OR, USA). The cells were counted using a Neubauer counting chamber (Hämocytometer, Merck, Darmstadt, Germany). The freshly isolated cells were directly sprayed and pipetted according to the experimental setups. Cell culture followed as set out below.
Cell spray device
A device for cell spray application was prototyped by StemCell Systems GmbH (Berlin, Germany). The device consists of an electronically controlled pneumatic fluid distribution control unit for the adjustment of the fluid flow rate and the air flow, and a sterilizable cell spray head with a nozzle and a clamping fixture on a linear motor, holding a cell suspension-containing syringe (Figure 1). A disposable 2 ml syringe (B. Braun, Melsungen, Germany) containing 1 ml of the freshly isolated cell suspension is connected via a Luer-lock tube with the spray nozzle. The fluid-flow of the cell suspension and the airflow in the spray nozzle are controlled. An airflow of 3,7 l/min and a fluid-flow of 4,2 cm3/min were used for all cell spray applications since prior experiments demonstrated that such adjustment did not affect the cells viability [17]. This adjustment results in a standardized spray pressure of 8,2 mmHg that was used in all experimental setups.
Figure 1: An early experimental cell-spray deposition device prototype developed for single skin cell suspension applications.
Cell spray deposition
The freshly isolated cells were suspended in a concentration of 7.5 x 105 – 1.8 x 106 cells/cm3 in M254 media. For the co-cultures, subsequently, 1 ml cell suspension was pipetted into 25 cm2 Petri dishes (Falcon, Franklin Lakes, NJ, USA) as control and 1 ml was aspirated into a Luer-lock 2 ml syringe (B. Braun, Melsungen, Germany) for the cell spray device (see supra). This suspension was sprayed onto a tissue culture dish of 25 cm2 (FALCON, CORNING, Tewksbury, MA). A spray time of 20 sec with a distance of 6 - 10 cm and by an angle of 30 ° - 50 ° was used with the cells of passage 0° [7, 8]. An average of 1,2 x 106 cells (with a range of 0,2 x 106 to 1,8 x 106) was sprayed onto the surface of the dishes. For the monocultures, Petri dishes with HEKs n=8, and MCs n=8 were sprayed and compared in follow-up cultures with the pipetted control cultures. For the separation of MCs from HEKs and the general cell culture see below.
Separation of HEKs and MCs / cell culture after spraying and pipetting
To also isolate HEKs and MCs as single cells, 1 ml cell suspension obtained from autologous epidermis was incubated on collagen-coated flasks (BD BioCoat Collagen I, BD Biosciences, Bedford, USA) for 15 min. Afterward, the supernatant that mainly contained MCs was transferred to small uncoated dishes (Becton Dickinson, Heidelberg, Germany) [17]. The adherent HEK cells were washed with PBS and then cultivated in serum-free and calcium-free EPILIFE Medium (Cascade Biologics, Inc., Portland, OR, USA) according to the method of Rheinwald and Green with minor modifications by Johnen et al. [16, 18-20]. For all cultures of the study, every third day the medium was changed. Only in a few cases, e.g., when the proliferation rate stagnated, 1 ml fresh medium was added to the cultures, so to maintain the cells’ milieu and still provide for sufficient nutrition.
When 70% confluence of cells was reached, they were split off with 5 ml 0,05% Trypsin for MCs and 5 ml 0,25 % Trypsin in case of HEKs. At this point, MCs were kept for no more than 2 min at room temperature (RT), preferably under the microscope, so to watch the cells detach and to stop the reaction, accordingly. HEKs were incubated for 5 min at 37°C with 0,25% Trypsin and stopped with FCS. Centrifugation followed by 1000 rpm (216 g) for 5 min, so to obtain a respective cell pellet. After centrifugation, only one-third of the pellet was seeded onto a new surface to continue culturing in another passage.
Biochemical analyses
The supernatant of unconditioned media was tested for baseline values. 2 ml samples were taken by a 1000 µl pipette (Eppendorf, Hamburg, Germany) during routine media change on the third, fifth and seventh day and analyzed at the central laboratory of our Charité, Campus Virchow-Clinic using a Roche COBAS FARA analyzer (Roche Diagnostics Deutschland GmbH, Mannheim, Germany). LDH, glucose, and lactate were chosen as a comprehensive parameter for evaluation of cell status, proliferation, and metabolism.
Morphology assessment
By comparison of a sprayed monoculture of HEKs and MCs in 25 cm2 Petri dishes and a conventionally pipetted control monoculture in flasks, we tested for differences regarding cell morphology and culture behavior up to passage 4°, individually. The experimental culture groups were first investigated by light microscopy (Zeiss, Axiovert 25, Göttingen, Germany) 24 hours after incubation and follow-up cultures were further observed until day 17 – when possible. The sprayed and conventionally pipetted cells were analyzed and compared concerning the morphologic appearance, adhesion and migration behavior. Also, cell spreading, and cell density were investigated for each cell type. Reaching 70% to 80% of confluence in one of the compared Petri dishes, defined the endpoint of experimental trials. Cell and culture morphology were documented by representative photographs using a Zeiss Axiovert 200M equipped with a QImaging Retiga 2000R (QImaging, Burnaby, Canada), and the software Image-Pro Plus 6.2 (Media Cybernetics Inc., Silver Spring, MD).
Single immunofluorescence / double immunofluorescence
Following routine cell isolation, HEKs and MCs were seeded in passage 0° on collagen I coated 8-well chamber slides (Nunc Lab-Tek Chamber Slide system, Sigma-Aldrich, St. Louis, MO) for single and double-immunofluorescence experiments. For monocultures, the appropriate medium was used for HEKs, i.e., EPILIFE, and for MCs, i.e., M254 medium. In the double-immunofluorescence setups, however, only M254 medium was used. The reason for this media selection is that both HEKs and MCs tolerated M254 during pre-experiments, but the successful cultivation of MCs was not possible in EPILIFE (data not shown). When the cells reached 70% to 80% confluence, we took off the chamber and cleaned the slides with PBS (PBS; PAA Laboratories, Cöelbe, Germany) containing Tween-20 (Sigma-Aldrich, Taufkirchen, Germany). Cells were fixed with 2% paraformaldehyde for 10 min at RT and permeabilized using 80% dry ice-Methanol at -20°C for additional 20 min. The following steps were performed at RT: The fixed cells were treated with a blocking buffer (Sigma-Aldrich, Taufkirchen, Germany) for 60 min. Afterward, antibodies were applied as follows: Primary antibodies for MCs were undiluted monoclonal anti-human melanoma antibody clone NKI/beteb raised in mouse (SANBIO Deutschland GmbH, Beutelsbach, Germany). Polyclonal anti-human cytokeratin 14 (CK14) raised in rabbit (dianova GmbH, Hamburg, Germany) were the primary antibodies for HEK staining with a dilution of 1:200. We further chose the following secondary antibodies for our study. The Alexa Fluor highly cross-adsorbed 488 nm goat/anti-mouse IgG Kit (Invitrogen GmbH, Karlsruhe, Germany) in a dilution of 1:1000, as binding partner with NKI/beteb for staining MCs, and the Alexa Fluor highly cross-adsorbed 594 nm goat/anti-rabbit IgG Kit (Invitrogen GmbH, Karlsruhe, Germany) in a dilution of 1:1000, as binding partner for CK14 for staining HEKs. All antibodies were incubated for one hr. The nucleus was stained with undiluted 4',6-Diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich, St. Louis, MO) for 20 min. Optionally, the cell slides were mounted with Aqua Poly/Mount (Polysciences, Inc., Warrington, PA) and sealed with nail polish.
Clinical case report
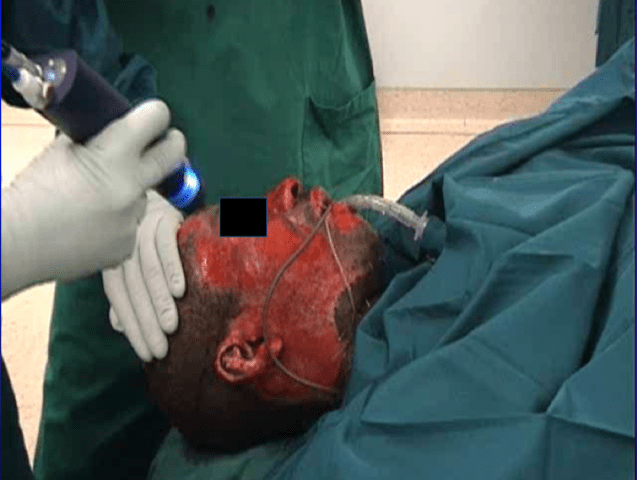
Photos of one clinical case treatment from one Afro-American patient with dark skin pigmentation are reported in (Figure 10), where the cell isolation method described here was clinically used to obtain an autologous skin cell preparation for immediate on-site cell spray-grafting (first cell suspension, passage 0°). This study was performed in Berlin, Germany, after the patient experienced a gas explosion trauma, within the regulatory framework of an Innovative Practice approach. Figure 10a shows the severe second-degree wound areas on day 11 in the face, post debridement, while the skin cell spraying is performed. The photo also reveals areas in the neck that represented third-degree burns, which were treated with split skin grafting, but where the cell graft was additionally sprayed into the gaps between the mesh. Information on the cell isolation and the clinical procedure is given in [7, 8, 14, 15].
Statistics
Data are expressed as mean values and standard deviation (SD). Cell counting of each sample was performed three times using a Neubauer counting chamber and was averaged to improve accuracy. Due to small sample sizes, a normality-distribution of data could be not assumed, and as a consequence, a non-parametric test was used. The groups were compared performing the unpaired Mann-Whitney-U-Test. Significance was defined by p ≤ 0.05. Morphology evaluation and culture appearance, as well as culture behavior, were descriptive. The expression of analyzed antibody markers was compared semi-qualitatively.
Results
Morphology assessment
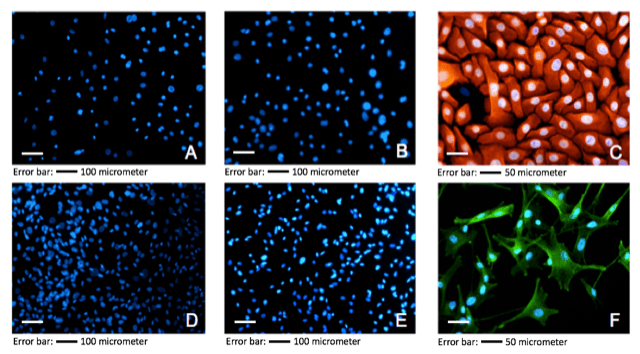
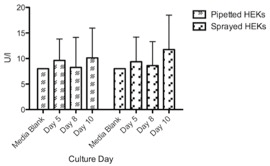
While time to reach 70% to 80% confluence averaged from 3 to 5 days, variations were observed from cells isolated from different donors (data not shown). Also, cell counts after isolation exhibited differences among the n=8 donor biopsies dependent on donor medical history (data not shown). The ratio of HEKs : MCs in our primary cultures were microscopically counted (n=8) as mean +/- SD 1,211,000 (+/- 574,343) HEK : 99,625 (+/- 59,025) MC; i.e., a ratio of approx. 12 : 1. The HEK follow-up monocultures exhibited its typical cobble-stone pattern with a homogenous appearance in culture (see Figure. 2C). The MC monocultures exhibited its typical multi-dendritic form with incorporated granule (including mature melanosomes), homogenous in appearance, during culture (see Figure 2F). Moreover, culture spreading of both cell types was normal; no delay or stagnation could be detected.
Figure 2: Immunostaining: monoculture of sprayed HEKs and MCs. A: Blank Staining HEKs (50x); B: Negative Control HEKs (50x); C: Positive Control HEKs (CK14+; 200x); D: Control (blank) Staining MCs (50x); E: Negative Control MCs (50x); F: Positive Control MCs (NKI/beteb+; 200x). The bar added indicates magnification.
Immunofluorescence for monoculture
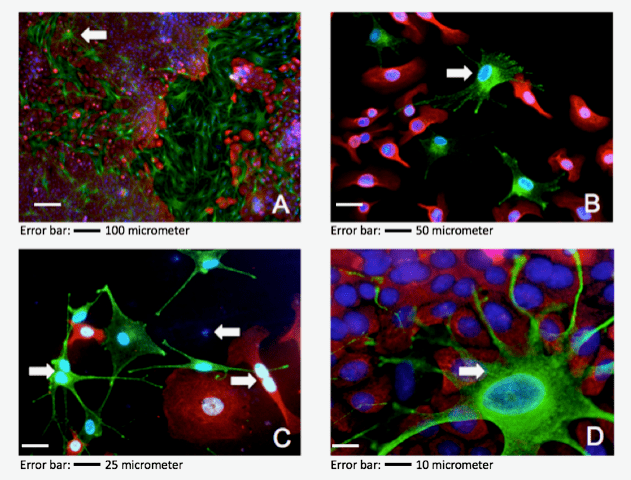
The color patterns of immunofluorescence staining (n=8) of sprayed and conventionally pipetted cells exhibited no remarkable differences (Figure 2). Monocultures of MCs and HEKs showed expected immunofluorescence signals concerning the specific antibodies applied. No cross staining was observed for NKI/beteb and K14+ antibodies showing specific staining for MCs and HEKs, respectively (Figures 2 A, B, D, E). Throughout the power fields investigated, red stained CK14+ HEKs could be observed, specified by respective blue DAPI staining of nuclei exhibiting the typical cobblestone pattern with homogeneous appearance (Figure 2C). Green stained NKI/beteb+ MCs could be observed where specific antibody attaches to intracellular melanosomes of an inner granule. The green stained MC monocultures exhibited the typical pattern of dendricity with homogenous appearance. The number of double-immunofluorescence stainings performed for freshly isolated HEKs and MCs of passage 0° was n=8. Red stained HEKs (CK14+) with interacting green stained MCs (NKI/beteb+) demonstrated under routine microscopy with different magnification effective co-culture for both conventionally pipetted and sprayed setups. Thereby, HEKs also exhibited its typical cobblestone pattern, and the MCs displayed multi-dendritic appearance. Figure 3A shows confluent co-culture at 50x magnification. In the left field, scattered green NKI/beteb+ stained MCs show red stained CK14+ HEKs intermittent. This figure reflects the in vivo situation in the healthy epidermal skin, namely 35 - 40 HEKs are supported by one MC [Eisinger and Marko, 1982] [21]. At 100x magnification, the specific dendricity of an MC connected to different HEKs was demonstrated; see white arrowheads in (Figure 3B). At a magnification of 200x, mitosis state of vital HEKs as well as for MCs could be observed; see white arrowheads in (Figure 3C).
Figure 3: Double immunofluorescence of sprayed HEKs / MCs suspension (representative power fields). A: 50x magnification of 80% confluent co-culture chamber slide of passage 0°, arrowhead indicates one MC cell surrounded completely by confluent HEKs; B: 100x magnification of a pre-confluent chamber slide of passage 0°, showing in vitro distribution of HEKs / MCs, one MC showing morphologic dendrites is indicated by an arrowhead, the granulation is shown by the green spotted pattern within the cell; C: 200x magnification, evident mitosis in MC cell (on the left) and HEK cell (on the right), mere blue spots indicate non-characterized cells; D: 400x magnification, detailed photo for MC and HEK interplay, the green spotted granulation pattern is highlighted by arrowheads.
Both cell types stuck in telophase. Figure 3D shows a single MC with surrounding confluent HEKs. Specifically, within the MC the typical granule pattern stained by green fluorescence is visible see white arrowheads in (Figure 3D). During the microscopic investigation, differences could be observed for the ratios of HEKs to MCs dependent on the biopsies from the isolated cells.
Glucose / lactate / LDH measurements
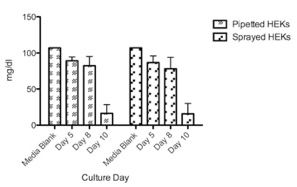
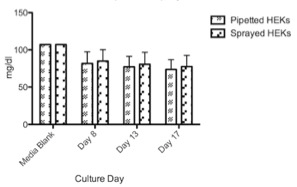
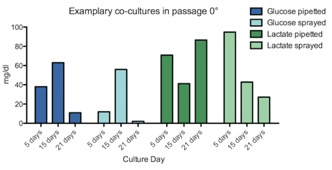
Cultured HEKs and MCs showed a similar glucose consumption pattern with no significant differences between sprayed and pipetted method (Figure 4 & 5). However, HEKs showed a higher glucose consumption rate where almost 75% of glucose was depleted after ten days while MCs only used 25% of the glucose in 17 days. The results suggest that HEKs have a higher expansion rate and metabolic activity compared to MCs. LDH liberation showed that the spray method did not inflict damage compared to pipetted cells, as cultured HEKs and MCs showed no significant differences between the two delivery methods (Figure 6 & 7). Feasibility of combined HEK-MC cell isolation for an anticipated intra-operative clinical cell spray application was assessed in vitro. As initial preliminary co-culture experiments of MCs and HEKs in EPILIFE medium and M254 medium showed after the first split to passage 1°, the MC cultured well in M254 but did not survive in EPILIFE medium; we showed only co-culture results obtained in the M254 medium. The metabolic activity showed a similar trend when comparing pipetted and sprayed methods (Figure 8). We only detected one exception with the lactate production of sprayed cells after 21 days with a value too low in comparison with the corresponding high glucose consumption. LDH liberation showed a similar trend between pipetted and sprayed cells (Figure 9), As a LDH liberation as a result of a damage occurs immediately after damage, the 21 day values are giving only an indirect information in regard to the LDH liberation behavior of the surviving cells.
Figure 4: Available glucose in medium (mg/dl) status of conventionally pipetted and sprayed HEKs in direct comparison over ten days investigation (n=8). No statistically significant differences could be revealed.
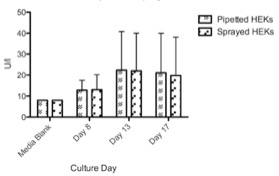
Figure 5: Available glucose in medium (mg/dl) of conventionally pipetted and sprayed MCs in direct comparison over 17 days investigation (n=8). No statistically significant differences could be revealed.
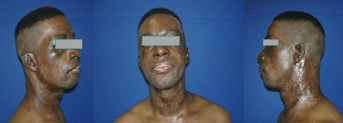
Case reports of patients in which the cell isolation method was clinically used under an innovative practice approach were already given previously, and we here present anecdotal photo documentation from an Afro-American patient with dark skin coloration [7, 8, 14, 15]. Satisfying results of this one patient with dark skin pigmentation suffering severe second-degree gas explosion burns are shown in (Figure 10). Figure 10a shows the patient’s face after wound debridement on day 11, during cell spray-grafting treatment of the second-degree areas on the face, while third-degree areas on the neck treated with mesh-grafting were also sprayed additionally into the open mesh areas with the cell spray-graft. Figure 10b indicates a satisfying re-pigmentation of the resulting wounds seven weeks post-treatment. The visible scar formation occurred in areas where mesh-grafting was performed additionally.
Figure 6: LDH liberation of conventionally pipetted and sprayed MCs in direct comparison over ten days investigation (n=8). No significant differences could be revealed.
Figure 7: LDH liberation of conventionally pipetted and sprayed MCs in direct comparison over 17 days investigation (n=8). No significant differences could be revealed.
Figure 8: The girl who was born with Down syndrome and an atrioventricular septal defect combined with a Tetralogy of Fallot. After hypoxic spells despite propranolol she had a modified Ballock Taussig Anastomosis at the age of 3 month. She suffered from severe heart failure due to pulmonary overcirculation and enhanced pulmonary pressure treated with frusemide, hydrochlorothiazide and spironolactone. Treatment with 2mg/kg propranolol leads to more pulmonary overcirculation, higher NT-BNP values and edema. We change to a lower propranolol dose of 0.5mg/kg together with digoxin to reduce the flow in the right ventricular outflow tract. HRV increased, heart rate and NT-BNP decrease. Systolic pulmonary artery pressure dropped down from 74mmHg to 20mmHg measured by Doppler echocardiography.
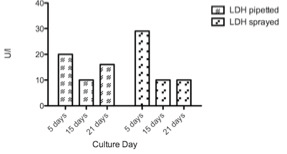
Figure 9: LDH liberation of conventionally pipetted and sprayed HEKs / MCs co-cultures of passage 0° (n=1); the bars represent different days of culture time (5 to 21 days).
Figure 10: Case results from an Afro-American patient with dark skin coloration, where the autologous skin cell isolation method for immediate spray grafting (passage 0°, primary cells) was clinically used in an innovative practice approach. FIG. 10a shows the patients face after wound debridement of a second-degree gas explosion burn during cell spray-grafting treatment of the second-degree areas in the face (the cell spray device shown in Figure 1 is visible), while third-degree burn areas on the neck were treated with mesh-grafting, but where the cell suspension spray-graft was also sprayed additionally into the open mesh areas. Figure 10b indicates a satisfying re-pigmentation of the resulting wound in the face and the neck seven weeks post healing. The visible scar formation occurred in areas where mesh-grafting was performed.
Discussion
During development, MCs derive from precursor stem cells, i.e., melanoblasts, situated in the neural crest [13]. In healthy skin, the brown pigment melanin is produced in MCs within the basal layer of the epidermis, deposited to melanosomes and finally transferred to the HEK via elongated dendrites. The melanin biosynthesis, also known as melanogenesis, takes place in the membrane-bound melanosomes of MCs and the relationship between MCs and HEKs is commonly referred to as the “epidermal melanin unit” (EMU), and the thought is that one MC is in contact with approx. 40 HEKs [21]. Accordingly, the functional interplay between MCs and HEKs is vital for developing and maintaining the appearance of the skin [1]. In addition to cosmetic aspects, melanin protects against ultraviolet radiation and is thus part of the body’s defensive system against external factors [1, 22]. Besides the conventional split-skin or meshed split-skin grafting, innovative clinical approaches are available, including autologous skin cell therapy with cultured cell sheets or single cultured cells for spray grafting [23-25]. Cultured sheets did not demonstrate a provision of MC, as the basal membrane-associated progenitors differentiate in culture expansion and passaged cells mainly provide adult keratinocytes [26]. Reconstructing the removed hypopigmented skin areas with either co-cultured autologous HEK-MC sheets (CEA) or with non-cultured HEK-MC transplantation was a therapeutic attempt for vitiligo; while costly this method was not fully satisfactory [3, 4, 25, 27-32]. Autologous non-cultured HEK-MC cell suspensions have been described in the clinical application for treatment of depigmented skin after burn trauma, but the outcomes varied [33].
We assessed the suitability of freshly isolated HEK and MC skin cells for immediate spray application, which was isolated according to a previously developed method [7, 14, 15, 32]. Our in vitro study compared mono- and co-cultures of passage 0° and follow-up cultures, to mimic combined cell isolation and spray application for intended clinical burn therapy. Considering the indirect means of biochemical data of glucose consumption, lactate production, and LDH liberation, morphologic phenotype, and proliferation data indicate that MCs can be transferred by spray deposition and behave in the follow-up co-cultures comparably to monocultures. Epidermal skin cells of n=8 different donor biopsies adhered well proliferated without any deceleration in time, and colonies were formed homogenously for HEKs and MCs in both, controls with conventionally pipetted cells and spray-deposited cells. Epidermal skin cells’ morphology was comparable in each case, and no impact by the spray procedure could be determined. Monocultures of HEKs and MCs derived from the same biopsies provided feasibility information for MC isolation and application.
We observed statistically not significant differences in the amount of granule depending on donor and on the biopsy location (data not shown). However, a clinical outcome of MC grafting likely also depends on individual patient disposition [16]. Although the HEK : MC ratio remains relatively constant among ethnic groups, the degree of skin pigmentation correlates with the degree of granulation, presenting melanin MCs located in the skin and the corresponding degree of the granule, that also depends on the body area location and sun exposure [22, 34, 35]. As a general strategy for achieving a desirable melanocyte density for desirable pigmentation with a graft, differences in melanocyte density of donor sites and the wound area to be treated should be considered beforehand. Since an individual prediction in skin color reflection of a graft appears problematic, a prediction of MC grafting outcome on final skin coloration may prove too complex. Constitutive skin pigmentation depends on so many factors, e.g., the melanin amount produced by MCs, its distribution, and to a lesser extent on MC density that such an approach might not be feasible [36, 37].
Our cell isolation characterization and primary cultures results showed a ratio of HEKs : MCs of approximately 12 : 1. As Todd et al., described ratios of HEKs to MCs in normal human of 35 : 1, and, e.g., Eisinger and Marko of 11 : 1 or Yamaguchi et al. of 10 : 1, we concluded that the method employed is suitable as an isolation method that provided both cell populations approximating the physiological ratio [21, 38, 39]. Moreover, our results demonstrate that skin cell isolation for obtaining mixed skin cell suspensions of vital and proliferating epidermal MCs together with HEKs is feasible; both cell types can be isolated without measurable impact on cell culture behavior. After experimental spraying, viable, proliferating and microscopically interacting HEKs and MCs were shown in passage 0° and follow-up cultures up to passage 4°. Using immunofluorescence, sprayed HEKs were distinguishable, and MCs attached in vitro showed a finely granulated cytoplasm. Biochemical parameter of sprayed skin cells and conventionally pipetted control cultures behaved as expected regarding glucose consumption and lactate metabolism as well as LDH liberation. Accordingly, no significant differences for biochemical parameter could be observed among the pipetted and sprayed cells (n=8).
Results from clinical use of the active cell isolation method in one case of an Afro-American patient with dark skin coloration suffering severe second-degree burns and performed under an innovative practice approach, support our work. Figure 10 indicates a satisfying re-pigmentation of the resulting wound seven weeks post application. The results obtained from this single case can only give anecdotal data and is not sufficient for conclusions on a clinical outcome. Nevertheless, our in vitro results could be of interest for planning such a multi-center clinical study, involving autologous melanocytes in freshly isolated autologous skin cell suspensions for an on-site cell spray grafting in the same session as the wound debridement.
Using MCs in spray-graft suspensions could represent a promising approach for treating severe partial-thickness burns and innovative therapy developments that also aim to address cosmetic aspects.
Author Contributions
Jörn Plettig, Christa Johnen, and Jörg C. Gerlach designed and coordinated the work, developed methods and prototypes and wrote the manuscript. Stefanie Mückler and Kirsten Bräutigam developed methods. Nidal Toman and Bernd Hartmann provided skin biopsies. Roger Esteban-Vives and all authors supported the study with data analyses and provided scientific discussions.
Acknowledgements
We thank RenovaCare Inc. New York, NY for sponsoring some of the resources.
Conflict of Interest Statement
Jörg C. Gerlach owns shares of StemCell Systems GmbH, Berlin, Germany, which provided prototypes for the in vitro study and the clinical application and has a financial interest in RenovaCare that commercializes skin cell spray devices. StemCell Systems temporarily employed Jörn Plettig and Christa M. Johnen.
Abbreviations
approx. – approximately CEA – cultured epithelial autografts EMU – epidermal melanin unit FCS – fetal calf serum HEK(s) – human epidermal keratinocyte(s) LDH – lactate dehydrogenase MC(s) – melanocyte(s) PC – positive control (pipetted cells) RT – room temperature SD – standard deviation * Words in capital letters indicate trademarks throughout the text.
Article Info
Article Type
Original ArticlePublication history
Received: Thu 30, May 2019Accepted: Tue 25, Jun 2019
Published: Mon 12, Aug 2019
Copyright
© 2023 Jörg C. Gerlach. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.03.09
Author Info
C. Johnen B. Hartmann J. Plettig Jörg C. Gerlach K. Bräutigam N. Toman Roger Esteban-Vives S. Hubald
Corresponding Author
Jörg C. GerlachDepartments of Surgery and Bioengineering, McGowan Institute for Regenerative Medicine, University of Pittsburgh, 3025 East Carson Street, PA, Pittsburgh, PA 15203, USA
Figures & Tables

References
- Costin GE, Hearing VJ (2007) Human skin pigmentation: melanocytes modulate skin color in response to stress. Faseb J 21: 976-994. [Crossref]
- Kwon H, Liu PH, Lew DH, Nishimura E, Orgill DP (2008) Hair follicle melanocyte cells as a renewable source of melanocytes for culture and transplantation. Eplasty 8: e7. [Crossref]
- Mulekar SV (2005) Long-term follow-up study of 142 patients with vitiligo vulgaris treated by autologous, non-cultured melanocyte-keratinocyte cell transplantation. Int J Dermatol 44: 841-845. [Crossref]
- Iman A, Akbar MA, Mohsen KM, Ali F, Armin A et al. (2013) Comparison of intradermal injection of autologous epidermal cell suspension vs. spraying of these cells on dermabraded surface of skin of patients with post-burn hypopigmentation. Indian J Dermatol 58: 240. [Crossref]
- Vyas NS, Lawrence KL, Griffith JL, Hamzavi IH (2015) Autologous, Noncultured Epidermal Cell Suspension Grafting in the Management of Mechanically and Chemically Induced Leukodermic Scars. J Cutan Med Surg 19: 488-493. [Crossref]
- Allouni A, Papini R, Lewis D (2013) Spray-on-skin cells in burns: a common practice with no agreed protocol. Burns 39: 1391-1394. [Crossref]
- Gerlach JC, Johnen C, McCoy E, Brautigam K, Plettig J et al. (2011) Autologous skin cell spray-transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns 37: e19-e23. [Crossref]
- Gerlach JC, Johnen C, Ottoman C, Brautigam K, Plettig J et al. (2011) Method for autologous single skin cell isolation for regenerative cell spray transplantation with non-cultured cells. Int J Artif Organs 34: 271. [Crossref]
- Plettig J, Johnen CM, Brautigam K, Zeilinger K, Borneman R et al. (2012) Active wound dressing with artificial capillaries for temporary wound irrigation and skin cell supply. Artif Organs 36: 446-449. [Crossref]
- Johnen C, Chinnici C, Triolo F, Plettig J, Brautigam K et al. (2013) Phenotypical characterization of 6-21-week gestational age human dermis and epidermal cell isolation methods for in vitro studies on epidermal progenitors. Burns 39: 300-310. [Crossref]
- Plettig J, Johnen CM, Brautigam K, Knospel F, Wonne EC et al. (2014) Feasibility study of an active wound dressing based on hollow fiber membranes in a porcine wound model. Burns 41: 778-788. [Crossref]
- Wood FM, Giles N, Stevenson A, Rea S, Fear M (2012) Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell ® kit. Burns 38: 44-51. [Crossref]
- Cho EG, Bin BH, Choi H, Park PJ, Kang HH et al. (2014) Novel method for isolating human melanoblasts from keratinocyte culture. Pigment Cell Melanoma Res 27: 489-494. [Crossref]
- Esteban-Vives R, Choi MS, Young MT, Over P, Ziembicki J et al. (2016) Second-degree burn injuries with six etiologies treated with autologous noncultured cell-spray grafting. Burns 42: e99-e106. [Crossref]
- Esteban-Vives R, Corcos A, Choi MS, Young MT, Over P et al. (2018) Cell-spray auto-grafting technology for deep partial-thickness burns: Problems and solutions during clinical implementation. Burns 44: 549-559. [Crossref]
- Johnen C, Hartmann B, Steffen I, Brautigam K, Witascheck T et al. (2006) Skin cell isolation and expansion for cell transplantation is limited in patients using tobacco, alcohol, or are exhibiting diabetes mellitus. Burns 32: 194-200. [Crossref]
- Schlabe J, Johnen C, Schwartlander R, Moser V, Hartmann B et al. (2008) Isolation and culture of different epidermal and dermal cell types from human scalp suitable for the development of a therapeutical cell spray. Burns 34: 376-384. [Crossref]
- Rheinwald JG, Green H (1975) Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell 6: 317-330. [Crossref]
- Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6: 331-343. [Crossref]
- Rheinwald JG, Green H (1977) Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 265: 421-424. [Crossref]
- Eisinger M, Marko O (1982) Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A 79: 2018-2022. [Crossref]
- Hendi A, Brodland DG, Zitelli JA (2006) Melanocytes in long-standing sun-exposed skin: quantitative analysis using the MART-1 immunostain. Arch Dermatol 142: 871-876. [Crossref]
- Atiyeh BS, Costagliola M (2007) Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns 33: 405-413. [Crossref]
- Fang T, Lineaweaver WC, Sailes FC, Kisner C, Zhang F (2014) Clinical application of cultured epithelial autografts on acellular dermal matrices in the treatment of extended burn injuries. Ann Plast Surg 73: 509-515. [Crossref]
- Navarro FA, Stoner ML, Lee HB, Park CS, Wood FM et al. (2001) Melanocyte repopulation in full-thickness wounds using a cell spray apparatus. Journal Burn Care Rehabil 22: 41-46. [Crossref]
- Esteban-Vives R, Young M, Over P, Schmelzer E, Corcos A et al. (2015) In vitro keratinocyte expansion for cell transplantation therapy is associated with differentiation and loss of basal layer derived progenitor population. Differentiation 89: 137-145. [Crossref]
- Pianigiani E, Andreassi A, Andreassi L (2005) Autografts and cultured epidermis in the treatment of vitiligo. Clin Dermatol 23: 424-429. [Crossref]
- El-Zawahry BM, Zaki NS, Bassiouny DA, Sobhi RM, Zaghloul A et al. (2011) Autologous melanocyte-keratinocyte suspension in the treatment of vitiligo. J Eur Acad Dermatol Venereol 25: 215-220. [Crossref]
