Adverse Effects of Non-Invasive Ventilation in Chronic Heart Failure: Keys to Avoid Complications
A B S T R A C T
Non-invasive mechanical ventilation (NIMV) is a therapeutic procedure that aims to supplement or improve ventilatory function. In recent decades, its benefit and multiple indications, have been demonstrated in the treatment of patients with chronic heart failure (CHF) and its acute complications [1]. However, the use of this therapy, in patients with chronic heart failure is not exempt from risks and complications, so it must be carefully individualized and requires close monitoring when ischaemic heart disease and/or arterial hypotension also coexist. Special mention requires the revision of the indication of servo ventilation in patients with severe chronic heart failure, due to its possible adverse effects.
Keywords
Non-invasive mechanical ventilation, chronic heart failure, hypotension, ischaemic heart disease, servo ventilation
Introduction
Nowadays, heart failure (HF) is a common public health problem with a high morbidity and mortality in our society. It´s difficult to precise the amount of people affected, due to the lack of studies. However, the estimated prevalence in Spain is about 5-6% of population, proportionally increased in elderly people and becoming greater than 15% in patients over 75 years [2]. HF is defined as a clinical syndrome in which the heart is unable to pump blood to the rest of the organism owing to the impairment of ventricular filling or ejection. The goal of the therapy is to reduce symptoms, to improve quality of life and also treat comorbidities such as atrial fibrillation, cardiovascular risk factors (hypertension, hypercholesterolemia, and diabetes mellitus) or sleep-related breathing disorders which worsen prognosis [3].
Pharmacologic treatment is the cornerstone of HF therapy; nonetheless, other therapies may help those patients, for instance cardiac rehabilitation, device therapy (as cardiac resynchronization therapy, implantable cardioverter-defibrillator) or non-invasive ventilation. Traditionally, non-invasive ventilation has been used in acute HF to reverse pulmonary edema, but it could have a role in chronic heart failure as a treatment for respiratory sleep disorders [4, 5].
Chronic Heart Failure and Adaptative Servo Ventilation
Respiratory disorders related to sleep (RDS) are found in a high number of patients with heart failure, and can be manifested as obstructive, central (Cheyne-stokes respiration) or mixed events [6]. The prevalence in the general population, according to the Sleep Heart Health Study cohort was 9%. Central sleep apneas, usually manifested as Cheyne-stokes respiration is characterized by episodes of apnea followed by an increase in respiratory rate and tidal volume, being detected mainly in patients with heart failure with depressed left ventricle ejection fraction (less than 45%) [7]. Although these respiratory events can be seen with some frequency in hearth failure, are not exclusive to this disease, and can be seen, for example, in neuromuscular disorders [8].
However, the presence of RDS increases the severity of heart failure and cardiac dysfunction, being an independent risk marker of poor prognosis and mortality in these patients. This fact is a consequence of increased sympathetic activity, facilitating diastolic dysfunction during apnea [9]. Taking all this into account, in obstructive sleep apnea with an apnea and hypopnea index (AHI) equal to or greater than 30, it could be treated depending on carbon retention, and several factors such as patient adaptation, among others, with continuous positive airway pressure (CPAP), bilevel positive airway pressure (BIPAP) or adaptive servo ventilation with the goal of improving sleep quality, ejection fraction and functional grade.
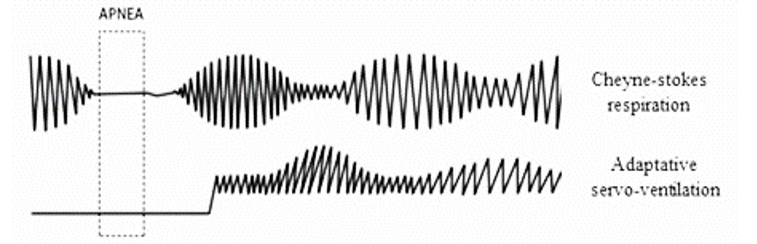
Servo ventilation consists of a ventilatory mode that offers an expiratory positive airway pressure (EPAP) and an inspiratory positive airway pressure (IPAP) depending on the detection of central apneas. The IPAP is automatically adjusted according to the respiratory effort and the EPAP prevents the collapse of the airway when patient does apnea (Figure 1) [6].
Figure 1: Adaptative servo ventilation recognises apneas and increases IPAP.
The therapy of choice in patients with central apneas is CPAP. Once the CPAP treatment has started, if the apnea control is insufficient, the adaptive servo ventilator appears to be the alternative therapy. The ASV improves symptoms, reduces the score in the Epworth test and allows better adaptation with greater therapeutic compliance (greater than 5 hours per night) compared to CPAP [10]. Previous reviews of this topic and meta-analysis of studies published before June 2010 indicated that ASV significantly improves LVEF and decreases AHI more than CPAP.
But a multicenter study with 1,324 patients and a follow-up for 64 months published in 2016 shows results totally unexpected: an increased mortality in those patients who received adaptive servo ventilation compared to the control group, with no benefit in terms of improvement in quality of life and symptoms, in spite of control of respiratory disorders and the oxygen desaturation index with adaptive servo ventilation [3]. This study included patients with heart failure, reduced ejection fraction (EF ≤ 45%) and an AHI of 15 or more events/hour, 50 per cent of these were central apneas. The patients followed the treatment of heart failure according to guidelines and were randomized to receive adaptive servo ventilation added to conventional therapy vs. medical treatment (control group). The justification of these results is uncertain, although it seems that the cause involved would be the elimination of the compensatory mechanism (in this case Cheyne-stokes respiration) that arises in heart failure. We cannot extrapolate these data to patients with heart failure with preserved ejection fraction or to patients with obstructive sleep apnea. The results cannot be extended to the use of devices other than those used in the study for adaptive servo ventilation [3].
In conclusion, authors do not recommend this ventilatory mode in patients with heart failure with a reduced ejection fraction, since it does not provide benefits and does increase cardiovascular and total mortality. However, more studies are necessary to assess the validity of these results and to evaluate if there are more complications that have not been described so far.
Chronic Heart Failure, Arterial Hypotension and NIMV
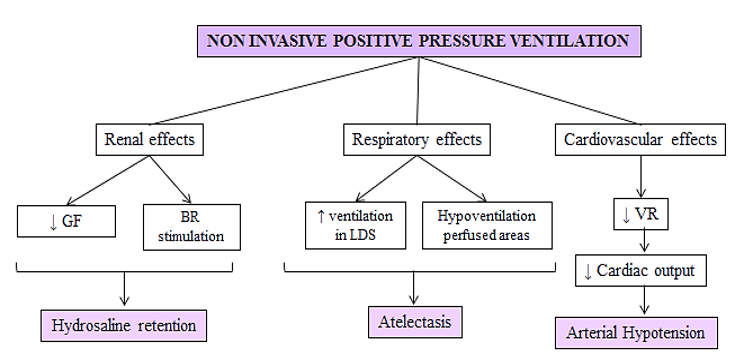
NIMV therapy has some important and significant benefits in patients with heart failure. This therapeutic method has contributed to improve the outcome of many pulmonology and cardiovascular diseases but the application of positive intrathoracic pressure through NIMV causes changes at different levels. On the other hand, it can increase ventilation in dead space areas and hypoventilates more perfused areas, due to differences in alveolar compliance. This involves alterations in the ventilation-perfusion ratio (V/Q), alveolar distension and atelectasis due to hypoventilation of the most perfused areas [11].
At cardiovascular level, hypotension during NIMV is usually attributable to diminished central venous blood return to the heart secondary to elevated intrathoracic pressures. It decreases venous return and therefore cardiac output, causing hypotension. In addition, it increases pulmonary vascular resistance, affecting right ventricular function (Figure 2). These patients are usually polymedicated with various hypotensive drugs for chronic heart failure (betablockers, angiotensin-converting enzyme inhibitors, diuretics). The application of the NIMV must be careful avoiding initially high pressures and it is necessary that this therapy is subjected to close monitoring due to the risk of a marked decrease in the blood pressure figures.
Figure 2: BR (baroreceptors), GF (glomerular filtration), LDS (lung dead space), VR (venous return).
On the other hand, hydrosaline retention is generated by different mechanisms such as the reduction of glomerular filtration or the stimulation of baroreceptors. These changes are also proportional to the applied pressure [11]. Elevated positive intrathoracic pressure produced by NIMV reduces venous return, with the consequent decrease in cardiac output and renal hypoperfusion. This leads to the activation of the renin-angiotensin-aldosterone system, increasing sodium reabsorption to compensate arterial hypotension. Therefore, in patients presenting a reduced ejection fraction, decompensation of their chronic heart failure can be developed.
Chronic Heart Failure, Ischaemic Cardiomyopathy and NIMV
NIMV has demonstrated to be beneficial for the treatment of obstructive sleep apnea (OSA) and its cardiovascular complications, reducing the risk of acute myocardial ischaemic and its recurrences, and cardiac arrhythmias in these patients [9]. Some studies describe an increased number of acute myocardial infarctions attributed to coronary vasoconstriction due to a rapid correction of blood oxygen levels, secondary to treatment with NIMV [4].
However, these studies are subject to a series of biases such as sample size or the presence of confounding factors (intrinsic characteristics of patients that make them more susceptible) that limit the validity of these results [5]. Therefore, more conclusive scientific studies would be necessary to confirm or discard these findings.
Conclusion
NIMV is a proven therapy for chronic heart failure and its complications, but it is not without risks. Due to its reducing effect on venous return and therefore on cardiac output, it can cause a drop-in blood pressure, especially in patients with hypotensive treatments. Recent studies have warned of an increase in mortality in patients with depressed left ventricle ejection fraction who received treatment with adaptive servo ventilation. Nevertheless, more studies are needed to reliably confirm or rule out the risk of acute myocardial infarction and other cardiovascular complication related to NIMV treatment. To conclude, NIMV treatment in chronic respiratory failure requires the ability of clinicians to both the appropriate patient candidate, but more studies are required.
Conflicts of Interest
None.
Article Info
Article Type
Review ArticlePublication history
Received: Fri 11, Dec 2020Accepted: Sat 26, Dec 2020
Published: Fri 08, Jan 2021
Copyright
© 2023 Laura Anoro Abenoza. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2021.01.04
Author Info
Laura Anoro Abenoza Buisán Esporrín Cristina
Corresponding Author
Laura Anoro AbenozaRespiratory Medicine Consultant, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain
Figures & Tables
References
- Uña Orejón R, Ureta Tolsada P, Uña Orejón S, Maseda Garrido E, Criado Jiménez A (2005) Noninvasive ventilation. Rev Esp Anestesiol Reanim 52: 88-100. [Crossref]
- Rodríguez Artalejo F, Banegas JR, Guallar Castillón P (2004) Epidemiology of heart failure. Rev Esp Cardiol 57: 163-170. [Crossref]
- Yang H, Sawyer AM (2016) The effect of adaptive servo ventilation (ASV) on objective and subjective outcomes in Cheyne-Stokes respiration (CSR) with central sleep apnea (CSA) in heart failure (HF): A systematic review. Hear Lung 45: 199-211. [Crossref]
- Ho KM, Wong K (2006) A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: A meta-analysis. Crit Care 10: R49. [Crossref]
- Peter JV, Moran JL, Phillips Hughes J, Graham P, Bersten AD (2006) Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet 367: 1155-1163. [Crossref]
- Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP et al. (2015) Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med 373: 1095-1105. [Crossref]
- Giannoni A, Raglianti V, Taddei C, Borrelli C, Chubuchny V et al. (2019) Cheyne-Stokes respiration related oscillations in cardiopulmonary hemodynamics in patients with heart failure. Int J Cardiol 289: 76-82. [Crossref]
- Deak MC, Kirsch DB (2014) Sleep-disordered breathing in neurologic conditions. Clin Chest Med 35: 547-556. [Crossref]
- Yoshihisa A, Suzuki S, Miyata M, Yamaki T, Sugimoto K et al. (2012) ‘A single night’ beneficial effects of adaptive servoventilation on cardiac overload, sympathetic nervous activity, and myocardial damage in patients with chronic heart failure and sleep-disordered breathing. Circ J 76: 2153-2158. [Crossref]
- Gallegos MB, Gómez T, Troncoso MF, Cabarcos R (2013) Características clínicas y polisomnográficas de pacientes con apnea central. ¿Se benefician de servoventilación adaptativa? Rev Patol Respir 16: 42-45.
- Gallardo Romero JM, García TG, Sancho Chust JN, González Martínez M (2010) Ventilación no invasiva. Arch Bronconeumol 46: 14-21.
