Journals
A tyrosine kinase Src; regulator of bone homeostasis
A B S T R A C T
The balance of bone formation by osteoblasts and bone resorption by osteoclasts is important for bone homeostasis. Pathologic high activity of osteoclasts and repression of bone osteoblastic bone formation result in bone metabolic diseases such as periodontal disease. To understand the mechanism of osteoblast and osteoclast function leads to establishment of therapy for bone metabolic disease. A tyrosine kinase Src deficient mice shows osteopetrosis because of defect bone resorbing activity and acceleration bone formation. This indicates Src is a key molecule to regulate bone resorption and bone formation. We discuss about the role of Src in osteoclast and osteoblast and entire bone metabolism.
Keywords
Src, Osteoclast, Osteoblast.
I N T R O D U C T I O N
Bone formation by osteoblast and bone resorption by osteoclasts are coordinately regulates bone homeostasis [1]. Accentuation of bone resorption compared with bone formation results in metabolic bone disease such as osteoporosis, rheumatoid arthritis and periodontitis [1-3]. Abnormal bone formation by osteoblast due to tooth ankylosis in dental field [4]. Thus, the normal activity balance between osteoclasts and osteoblasts are important in health promotion. The tyrosine Src deficient mice show osteopetrosis because of decreased osteoclastic bone resorption activity and osteoblastic bone formation acceleration [5-7]. This indicates Src is one of the important regulators of bone homeostasis. Understanding of the molecular role of Src will develop methods of treatment for the bone metabolic disease.
The role of Src in osteoclasts
Src plays important role in cell proliferation, cell growth, cell spreading and cell migration [7-11]. However, only phenotype Src deficient mice have is osteopetrosis because of defect of bone resorption and activation of bone formation [6, 7]. Src has 8 family members and these family members may complement the role of Src in many tissues. The expression and activation of Src is strictly regulated lower level by several systems in many tissues [12-19]. On the other hand, expression level and activation is very high in osteoclasts [20]. This shows there is unique regulatory mechanism of Src in osteoclasts. In many tissues, c-terminal Src kinase (Csk) phosphorylates the tyrosine in Src c-terminal and negatively regulates Src kinase activity [14]. Even though Csk is expressed as much as other tissue, Src activity is highly regulated in osteoclasts. Src is localized around cell membrane by myristoylation or palmitoylation of its n-terminal. On the other hand, Csk is ubiquitously localized in cytoplasm because Csk does not have transmembrane domain. Phosphoprotein membrane anchor with glycosphingolipid microdomains 1 (PAG) / Csk binding protein (Cbp) binds Csk and recruits Csk when Csk inhibits Src activity [21]. In osteoclasts, Cbp expression is suppressed by Receptor activator NF-κB ligand (RANKL) during osteoclast differentiation. As the results, Csk cannot be localize in cell membrane and regulate Src activity [16]. In recent study, Src localization is regulated by protein phosphatase 1 regulatory protein 18 (PPP18) and Protein phosphatase 1 (PP1) complex through dephosphorylation of Serine residue of Src n-terminal domain [17]. Actin ring formation and bone resorbing activity of osteoclasts are suppressed due to separation of Src from cell membrane by PPP1r18 and PP1 complex [17].
Osteoclasts loss attachment to bone matrix at sealing zone and acidic environment to bone resorption, although osteoclasts are differentiated and survive in Src deficient mice. Moreover, Src deficient osteoclasts did not form a characteristic actin structure, actin ring corresponding with sealing zone because of disturbance of actin organization in vitro [16, 22]. This indicate Src regulates actin organization although Src does not have actin binding domain [10]. Thus, it needs some actin regulatory proteins in regulation of actin ring formation by Src in osteoclasts. Src makes complex with many proteins such as Cortactin, p130Cas, c-Cbl, Cbl-b, Pyk2, Dynamin and Vav3 to promote actin ring formation [23-27]. Recently, an actin binding protein Plectin is reported as a Src binding and actin ring regulatory protein [28, 29]. These proteins are essential for actin ring formation and bone resorption.
The role of Src in osteoblasts and osteocytes
Src expression and activity is not so higher in osteoblasts and osteocytes than other tissues. However, bone formation by osteoblasts is promoted in Src deficient mice [5]. This result indicates Src has a specific role in osteoblasts. The transcriptional factor runt related transcription factor 2 (Runx2) is essential for osteoblast differentiation and plays master regulator of osteoblast differentiation and bone formation [30]. Runx2 localization to nuclear and transcriptional activity is inhibited by binding to Yes associated protein 1 (YAP) through YAP phosphorylation by Src [31]. This study indicates Src plays as inhibitory protein of Runx2 in osteoblasts. On the other hand, it is reported that Src phosphorylates Osterix, a transcriptional factor that is essential for osteoblast differentiation subsequent to Runx2 activation and up-regulates Osterix nuclear localization and osteoblast differentiation [32, 33]. Together, Src has functions both activation and inhibition of osteoblast differentiation. To think of the phonotype of Src deficient mice, other Src family kinase may be rescue Osterix nuclear localization but may not interrupt Runx2 activation.
Osteocytes are differentiated from osteoblasts and regulates balance of bone remodeling [1]. Osteocytes receive mechanical stress and regulates bone mass [34, 35]. Src suppresses anabolic gene expression in osteocytes under mechanical loading [36].
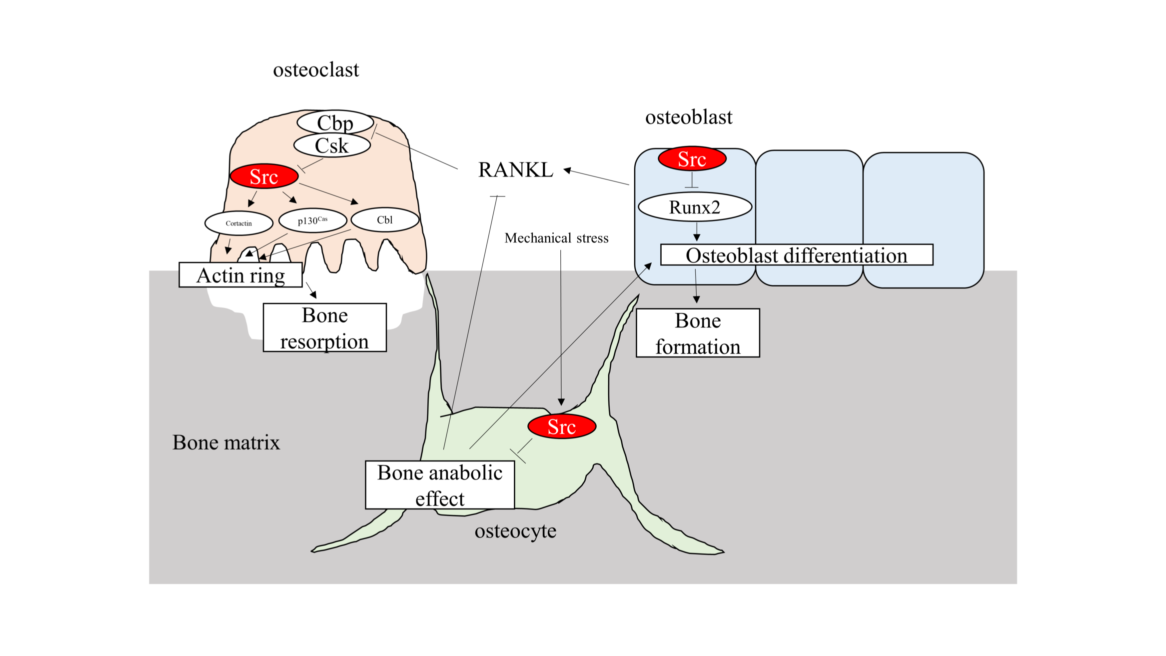
Altogether, Src promotes osteoclastic bone resorption and suppress osteoblast function (Figure). Regulation of Src function is one of the targets of therapy for periodontal disease and other bone metabolic disease. Src inhibitor is one of the candidates of treatment [37].
Figure 1: Schema of Src function in osteoclast, osteoblast and osteocyte. Src plays central role in osteoclastic bone resorption through actin organization. Src is a negative regulator of Runx2 transcriptional activity and osteoblast differentiation. Src also receives mechanical stress and regulates bone homeostasis in osteocyte.
Conflict of interest
All authors state that they have no conflicts of interest.
Acknowledgements Affiliations
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI 25670870 and 18K09509 to T.M.,).
Article Info
Article Type
Research ArticlePublication history
Received: Wed 30, Jan 2019Accepted: Fri 15, Feb 2019
Published: Thu 28, Feb 2019
Copyright
© 2023 Takuma Matsubara. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2019.01.003
Author Info
Shoichiro Kokabu Takuma Matsubara Tatsuki Yaginuma
Corresponding Author
Takuma MatsubaraDivision of Molecular Signaling and Biochemistry, Kyushu Dental University, 2-6-1, Manazuru, Kitakyushu, Fukuoka, 8038580, Japan
Figures & Tables
References
- Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19: 179-192. [Crossref]
- Graves DT, Oates T, Garlet GP (2011) Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol 3. [Crossref]
- Bartold PM, Marshall RI, Haynes DR (2005) Periodontitis and Rheumatoid Arthritis: A Review. J Periodontol 76: 2066-2074. [Crossref]
- Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, et al. (2005) Bisphosphonate Therapy Improves the Outcome of Conventional Periodontal Treatment: Results of a 12-Month, Randomized, Placebo-Controlled Study. J Periodontol 76: 1113-1122. [Crossref]
- Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, et al. (2000) Decreased C-Src Expression Enhances Osteoblast Differentiation and Bone Formation. J Cell Biol 151: 311-320. [Crossref]
- Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR (1992) Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest 90: 1622-1627. [Crossref]
- Soriano P, Montgomery C, Geske R, Bradley A (1991) Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64: 693-702. [Crossref]
- Schwartzberg PL (1998) The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene 17: 1463-1468. [Crossref]
- 9.Vlahovic G, Crawford J (2003) Activation of tyrosine kinases in cancer. Oncologist 8: 531-538. [Crossref]
- Boggon TJ, Eck MJ (2004) Structure and regulation of Src family kinases. Oncogene 23: 7918-7927. [Crossref]
- Ingley E (2008) Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta 1784: 56-65. [Crossref]
- Drobek A, Kralova J, Skopcova T, Kucova M, Novák P, et al. (2015) PSTPIP2, a Protein Associated with Autoinflammatory Disease, Interacts with Inhibitory Enzymes SHIP1 and Csk. J Immunol 195: 3416-3426. [Crossref]
- Superti-Furga G, Fumagalli S, Koegl M, Courtneidge SA, Draetta G (1993) Csk inhibition of c-Src activity requires both the SH2 and SH3 domains of Src. EMBO J 12: 2625-2634. [Crossref]
- Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H (1991) CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem 266: 24249-24252. [Crossref]
- Davidson D, Chow LM, Veillette A (1997) Chk, a Csk family tyrosine protein kinase, exhibits Csk-like activity in fibroblasts, but not in an antigen-specific T-cell lin. J Biol Chem 272: 1355-1362. [Crossref]
- Matsubara T, Ikeda F, Hata K, Nakanishi M, Okada M, et al. (2010) Cbp Recruitment of Csk Into Lipid Rafts is Critical to c-Src Kinase Activity and Bone Resorption in Osteoclasts. J Bone Miner Res 25: 1068-1076. [Crossref]
- Matsubara T, Kokabu S, Nakatomi C, Kinbara M, Maeda T, et al. (2018) The Actin-Binding Protein PPP1r18 Regulates Maturation, Actin Organization, and Bone Resorption Activity of Osteoclasts. Mol Cell Biol 38. [Crossref]
- Hakak Y, Martin GS (1999) Ubiquitin-dependent degradation of active Src. Curr Biol 9: 1039-1042. [Crossref]
- KF Harris, I Shoji, EM Cooper, S Kumar, H Oda, et al. (1999) Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc Natl Acad Sci U S A 96: 13738-13743. [Crossref]
- Horne WC, Sanjay A, Bruzzaniti A, Baron R (2005) The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev 208: 106-125. [Crossref]
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, et al. (2000) Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404: 999-1003. [Crossref]
- Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, et al. (2008) The Tyrosine Kinase Activity of c-Src Regulates Actin Dynamics and Organization of Podosomes in Osteoclasts. Mol Biol Cell 19: 394-404. [Crossref]
- Matsubara T, Myoui A, Ikeda F, Hata K, Yoshikawa H, et al. (2006) Critical role of cortactin in actin ring formation and osteoclastic bone resorption. J Bone Min Metab 24: 368-372. [Crossref]
- Uehara S, Udagawa N, Kobayashi Y (2018) Non-canonical Wnt signals regulate cytoskeletal remodeling in osteoclasts. Cell Mol Life Sci 75: 3683-3692. [Crossref]
- Tanaka S, Neff L, Baron R, Levy JB (1995) Tyrosine phosphorylation and translocation of the c-cbl protein after activation of tyrosine kinase signaling pathways. J Biol Chem 270: 14347-14351. [Crossref]
- Nakamura I, Jimi E, Duong LT, Sasaki T, Takahashi N, et al. (1998) Tyrosine phosphorylation of p130(Cas) is involved in actin organization in osteoclasts. J Biol Chem 273: 11144-11149. [Crossref]
- Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, et al. (2007) Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. J Cell Biol 178: 1053-1064. [Crossref]
- T Matsubara, M Kinbara, T Maeda, M Yoshizawa, S Kokabu, et al. (2018) A Cytolinker Protein , Plays an Important Role in Differentiation and Actin Ring Formation in Osteoclasts, Mathews. J Cytol Histol 1: 6-8.
- Matsubara T, Kinbara M, Maeda T, Yoshizawa M, Kokabu S, et al. (2017) Regulation of osteoclast differentiation and actin ring formation by the cytolinker protein plectin. Biochem Biophys Res Commun 489: 472-476. [Crossref]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, et al. (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755-764. [Crossref]
- Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, et al. (2004) Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J 23: 790-799. [Crossref]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, et al. (2002) The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 108: 17-29. [Crossref]
- Choi YH, Han Y, Lee SH, Cheong H, Chun KH, et al. (2015) Src enhances osteogenic differentiation through phosphorylation of Osterix. Mol Cell Endocrinol 407: 85-97. [Crossref]
- Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26: 229-238. [Crossref]
- Komori T (2013) Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res 352: 191-198. [Crossref]
- Hum JM, Day RN, Bidwell JP, Wang Y, Pavalko FM (2014) Mechanical loading in osteocytes induces formation of a Src/Pyk2/MBD2 complex that suppresses anabolic gene expression. PLoS One 9: e97942. [Crossref]
- Thouverey C, Ferrari S, Caverzasio J (2018) Selective inhibition of Src family kinases by SU6656 increases bone mass by uncoupling bone formation from resorption in mice. Bone 113: 95-104. [Crossref]
