A Simple and Convenient Method to Isolate and Culture Rat Aorta Endothelial Cells
A B S T R A C T
Aim: Endothelial injury results in vascular disorders. Culture of endothelial cells (ECs) is important to investigate the pathogenesis of vascular diseases. ECs of rat aorta are widely applied in these researches. However, there are many problems to isolate them, which make it hard to culture primary ECs. In the present study, we established a simple and convenient method to isolate and culture rat aorta ECs.
Methods: Two 5ml centrifuge tubes were prepared. An opening (3 mm × 25 mm) was made in one tube wall while the other was cut in half longitudinally. After the rat aorta was dissected along one side and attached to the wall of the half tube, the tube with an opening and the half tube with aorta were mated and secured by a rotund metal clip. Then, ECs were digested via the opening by 0.2% collagenase I at 37℃ for 1 h and identified by immunofluorescence of von Willebrand factor (vWF) and smooth muscle actin (SMA).
Results: A simple method for isolating ECs were established. Cultured ECs exhibited typical “cobblestone” morphology at confluence. vWF was positively expressed in the ECs, and neither the negative stain of vWF nor the positive stain of SMA was found.
Conclusion: This simple and convenient method to isolate and culture ECs of rat aorta can be implemented with readily available 5ml centrifuge tubes and will greatly benefit the research of vascular diseases.
Keywords
Endothelial cell, rat, aorta, smooth muscle cell, in vitro
Introduction
Vascular endothelial cells (ECs) cover the internal surface of the vessel and comprise the largest homogenous surface of the body [1]. They play important roles in homeostatic mechanisms, such as regulation of vascular tone, maintenance of a non-thrombotic environment, and mediating the immune defense [2]. ECs injury results in vascular disorders, even initiate the vascular injury [3, 4]. Hence the focus on ECs for the investigation of vascular diseases is very important [5, 6]. Culture of ECs is important in the research of pathogenic mechanism of cardiovascular diseases. Human umbilical vein endothelial cell (HUVEC) is the most commonly used to investigate vascular disorders because it is easy to obtain and culture [7]. However, ECs isolated from veins might not represent the disease process in artery.
ECs from rat aorta are widely used for research in angiocardiopathy such as hypertension and atherosclerosis [8]. Currently, enzymatic digestion and explants are commonly used to culture ECs [9, 10]. But the rat aorta has many small branches, and enzyme would escape from the aorta if it was perfused as HUVECs culture. If ECs are isolated through flattening of dissected aorta on the digestive solution, smooth muscle cells (SMCs) and other cells will be digested leading to the contamination of ECs due to the edge of the aorta being smeared the digestive solution. For cultured by explants of endothelium, ECs are easy to be contaminated by SMCs even if the explants were constantly removed after ECs grew from them. Besides, this is time-consuming and inconvenient [11]. Moreover, to remove SMCs, special equipment, such as magnetic beads combined with antibodies or fluorescence-activated cell sorter (FACS) is required [12]. The method described here is based on enzyme digestion, resulting in one simple tool. The procedure does not require special equipment such as magnetic beads or FACS, and a large number of ECs can be obtained in a short span of time by means of the method.
Methods
I Animals
Wistar Kyoto rats (male or female, 8 weeks of age) were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). All experimental procedures involving the use and care of animals were conducted in accordance with protocol guidelines approved by the Experimental Animal Committee of Chengde Medical University.
II Tool
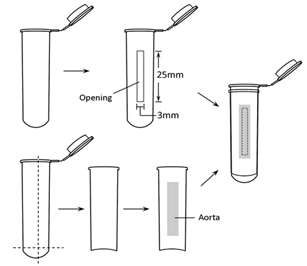
Two 5ml centrifuge tubes were prepared. A rectangle opening (3 mm × 25 mm) was made longitudinally on one tube wall and was carefully trimmed and edges smoothed. The second tube was split in half longitudinally, and with the round bottom part excised, a half tube wall was obtained. The tube with an opening, the half tube wall, and a rotund metal clip were sterilized by autoclaving (Figure 1).
Figure 1: Assembly of centrifuge tubes.
III Surgical Procedures
Rat was anaesthetized with intraperitoneal injection of pentobarbital sodium (30g/L, 50mg/kg of body weight). A thoracotomy was performed, and approximately 4 cm thoracic aorta was quickly excised. Adipose tissue around the aorta and adventitia were gently removed. The aorta was longitudinally dissected along one side by a straight ophthalmic scissors and rinsed three times by PBS.
IV Isolation and Culture of ECs
The dissected aorta was centrally attached to the wall of the half tube. The tube with an opening and the half tube with aorta were mated and secured by the rotund metal clip. Collagenase I (0.2 %, 200 μL; Life Technologies, Carlsbad, New Mexico, USA) was added to the intima via the opening and incubated for 1h at 37℃. After that, D-Hanks solution (1-1.5 ml) was added in the tube to remove ECs. ECs were removed from the aorta by gently flushing with D-Hanks solution, and collected by centrifugation at 1500 rpm for 5 min. Then the precipitate was gently re-suspended with endothelial cell medium (ECM; Sciencell, Santiago, California, USA), and cultured in a T25 flask.
V Culture of SMCs
The aorta that has been incubated with the collagenase was washed in PBS to remove the enzyme. After the intima was scraped away by a scalpel, pieces of aorta (1-2 mm each) were cut by an ophthalmic scissors, and put into a T25 flask, where these pieces were dried for 2h. Dulbecco’s modified Eagles’ medium (DMEM, Life Technologies) containing 10% fetal bovine serum (FBS, Life Technologies) was added gently.
VI Immunofluorescence Staining
Both ECs and SMCs were divided into control group and experiment group. All the cells were fixed with cold methanol for 10 min on a coverslip, and dried naturally at room temperature for 5 min. After blocking in 4% bovine serum albumin in PBS for 1h at room temperature, 2 μg/ml of rabbit antibody against von Willebrand factor (vWF; Santa Cruz Biotechnology, California, USA ) or 1 μg/ml of mouse antibody against smooth muscle actin (SMA; Santa Cruz) were incubated at 4℃ overnight in the experiment group. After washing with PBS three times, cells were incubated with TRITC-conjugated goat anti-rabbit (Santa Cruz) and FITC-conjugated rabbit anti-mouse IgG antibodies (Santa Cruz) at 37℃ for 30 min, then incubated with 4', 6-diamidino-2-phenylindole (DAPI; Beyotime Biological Technology Inc., Haimen, China) for 5 min. Cells were washed three times with PBS, mounted with antifade reagent. In the control group, cells were only incubated with TRITC-conjugated goat anti-rabbit and FITC-conjugated rabbit anti-mouse IgG antibodies.
Results
I Morphology of ECs and SMCs
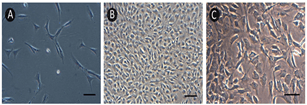
ECs was attached to the bottom of flask about 2h after seeded. Division and proliferation of ECs could be observed after 3 days (Figure 2A). It was about 10 days until ECs were confluent and formed “cobblestone” morphology (Figure 2B). SMCs presented circle or spindle shape and grew in “peak-valley” mode (Figure 2C).
Figure 2: The morphology of A) & B) ECs and C) SMCs of rat aorta. Scale bar: 50 μm.
II Immunofluorescence Analysis
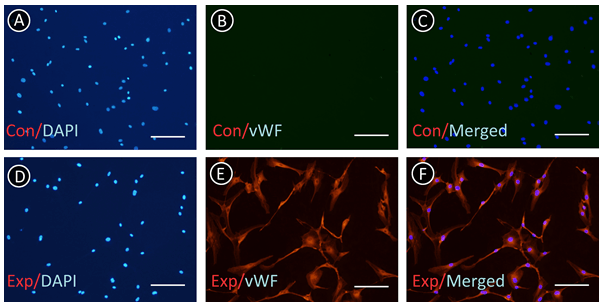
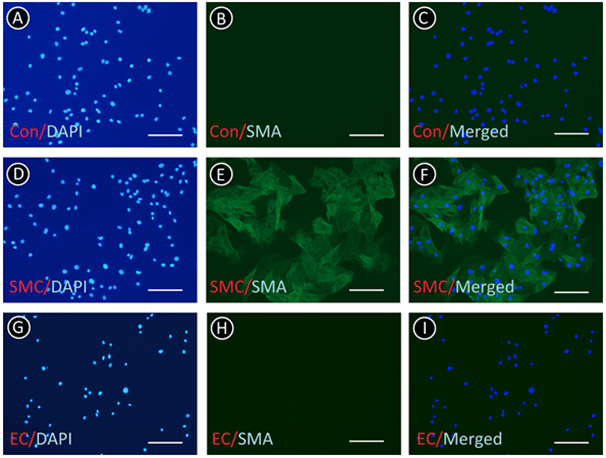
Immunofluorescence was employed to identify ECs and SMCs and determine whether ECs were contaminated by SMCs. As shown in (Figure 3), there was no negative stain of vWF in the ECs. From (Figure 4), we found that all of the SMCs presented the positive stain of SMA; however, there was no positive stain of SMA in ECs. These results indicated that the ECs were not intermingled with SMCs and other cells.
Figure 3: ECs were identified by immunofluorescence of vWF. A-C) Control group (Con) presented negative stain in the absence of primary antibody. D-F) Negative stain cell was not found in the experiment group (Exp). Scale bar: 50 μm.
Figure 4: ECs and SMCs were identified by immunofluorescence of SMA. A-C) Negative control of SMCs (Con) in the absence of primary antibody. D-F) SMCs were positive for SMA. G-I) ECs were not stained. Scale bar: 50 μm.
Discussion
ECs of large blood vessels such as human umbilical vein and aorta are commonly isolated and cultured by enzymatic digestion or explants. Highly purified ECs can be rapidly isolated from human umbilical vein by the enzyme perfused in the lumen. However, the rat aorta has lot of small branches, and the enzyme would leak out of the lumen if the aorta was directly perfused by digestive solution as human umbilical vein. If the dissected aorta is laid on the digestive solution, the edge of the aorta would be digested, and ECs will intermingle with SMCs and fibroblasts. Culture of explants can be implemented by directly pasting the pieces of aorta or the intima torn from aorta, even aortic rings on the bottom of the dish [13-15]. The above method is characterized by simple operation and suitable for isolating and culturing aortic ECs of small animals such as rat, mouse. However, it takes several days for ECs to grow out of the explants. In addition, once ECs are observed around an explant, the explant should be removed from the dish to reduce the contamination of SMCs. This is time-consuming and inconvenient. In addition, in most cases, the ECs cultured by explants need purification by special equipment because of low purity [16].
In this study, when ECs are cultured by the tool, both leakage of digestive solution and digestion of aorta edge are avoided. Moreover, it is not necessary to remove the explants constantly and purify with special equipment. A large number of primary ECs can be obtained in a short span of time, which simplifies the culture of aorta ECs. After repeated testing, we found that 5 ml centrifuge tube is the ideal material to make the culture tool because it is easily available, can readily be cut and sterilized by autoclaving. Furthermore, all of the digestive operations are conducted in the tube, which facilitates the collection of the digested cells. There are two critical points in executing the method. First, the aorta should fully cover the tube opening, in case the digestive solution leaks and digests other cells in the aortic cross-section. We suggest tubes with different opening sizes should be made to meet different operational requirements. Second, the time of digestion should be appropriate for different rat ages or species. For example, to isolate ECs from 3-4 weeks old rat, the opening of the tool should be smaller to fit the smaller aorta, and the digestive time should be shorter to avoid excessive digestion [17]. We believe that this simple and convenient method to isolate ECs from rat aorta is highly useful for the research of endothelium-related diseases.
Acknowledgment
The present study was supported by grants from Health Commission of Hebei Province (No. 20181150).
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 07, Dec 2020Accepted: Mon 28, Dec 2020
Published: Thu 21, Jan 2021
Copyright
© 2023 Fanxing Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2021.01.03
Author Info
Ruixiang Li Fanxing Meng Yonggang Zhao
Corresponding Author
Fanxing MengDepartment of Pathophysiology, Chengde Medical University, Chengde, China
Figures & Tables
References
- Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP et al. (2005) Circulating endothelial cells biomarker of vascular disease. Thromb Haemost 93: 228-235. [Crossref]
- Widlansky ME, Gokce N, Keaney JF Jr, Vita JA (2003) The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149-1160. [Crossref]
- Ruggiero D, Paolillo S, Ratta GD, Mariniello A, Formisano T et al. (2013) Endothelial function as a marker of pre-clinical atherosclerosis: assessment techniques and clinical implications. Monaldi Arch Chest Dis 80: 106-110. [Crossref]
- Tesfamariam B, DeFelice AF (2007) Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol 46: 229-237. [Crossref]
- Strisciuglio T, De Luca S, Capuano E, Luciano R, Niglio T et al. (2014) Endothelial dysfunction: its clinical value and methods of assessment. Curr Atheroscler Rep 16: 417. [Crossref]
- Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T (2005) A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb 12: 138-142. [Crossref]
- Lidington EA, Rao RM, Marelli Berg FM, Jat PS, Haskard DO et al. (2002) Conditional immortalization of growth factor-responsive cardiac endothelial cells from H-2K(b)-tsA58 mice. Am J Physiol Cell Physiol 282: C67-C74. [Crossref]
- Polovina MM, Potpara TS (2014) Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med 126: 38-53. [Crossref]
- Cole OF, Fan TP, Lewis GP (1986) Isolation, characterization, growth and culture of endothelial cells from the rat aorta. Cell Biol Int Rep 10: 399-405. [Crossref]
- McGuire PG, Orkin RW (1987) Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Lab Invest 57: 94-105. [Crossref]
- Tian H, Suo N, Li F, Yang CL, Qiong X (2014) An effective method of isolating endothelial cells from intact rat aorta. Cell Biochem Biophys 70: 423-427. [Crossref]
- Marelli Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI (2000) Isolation of endothelial cells from murine tissue. J Immunol Methods 244: 205-215. [Crossref]
- Gordon EL, Danielsson PE, Nguyen TS, Winn HR (1991) A comparison of primary cultures of rat cerebral microvascular endothelial cells to rat aortic endothelial cells. In Vitro Cell Dev Biol 27A: 312-326. [Crossref]
- Kreisel D, Krupnick AS, Szeto WY, Popma SH, Sankaran D et al. (2001) A simple method for culturing mouse vascular endothelium. J Immunol Methods 254: 31-45. [Crossref]
- Nicosia RF, Villaschi S, Smith M (1994) Isolation and characterization of vasoformative endothelial cells from the rat aorta. In Vitro Cell Dev Biol Anim 30A: 394-399. [Crossref]
- Zhang YH, Hu Y, Mei H, Wang HF, Guo T et al. (2007) Compared study for three methods to isolate endothelial cells from rat aorta (in Chinese). Chin J Microcirculation 17: 15-16.
- Pan LL, Dai M, Wang W (2007) A new method for culturing endothelial cells from the rat aorta (in Chinese). Chin Pharmacol Bull 23: 410-413.
