A Rare Case of Sino-Orbital Invasive Aspergillosis in a Kidney Transplant Recipient
A B S T R A C T
Invasive sino-orbital aspergillosis is an uncommon but potentially life-threating complication of kidney transplantation. Here we report a case of a patient with invasive aspergillus fumigatus sinusitis extending into the orbit in a kidney transplant recipient who was successfully treated with voriconazole and surgical debridement without requiring orbital exenteration. This case illustrates a rare but life-threatening complication of immunosuppression that highlights the importance of suspecting and promptly recognizing fungal infection of the sinuses in vulnerable organ transplant recipients in order to avoid significant morbidity and mortality.
Keywords
Invasive aspergillosis, sino-orbital, kidney transplant
Introduction
Sino-orbital aspergillosis is a rare disorder that results when infection of the paranasal sinuses spreads into the orbit. This form of invasive aspergillosis may present as localized disease, which spreads to adjacent structures, or as a fulminant systemic disease. The infection often originates from the maxillary sinus, followed by the ethmoid and sphenoid sinuses and rarely from the frontal sinuses. It is associated with high morbidity and mortality especially when the diagnosis is delayed or presents in the sino-orbit cranial form. Here, we describe a case of invasive Aspergillus fumigatus sinusitis extending into the orbit in a kidney transplant recipient who was successfully treated with voriconazole and surgical debridement.
Case Report
A 70-year-old Caucasian male, with past medical history significant for end stage renal disease secondary to focal segmental glomerulosclerosis status post pediatric en bloc kidney transplant, presented with nasal congestion, sinus pain and general malaise six months after transplant.
He was on maintenance immunosuppression therapy with tacrolimus, mycophenolate mofetil, and prednisone. Despite completing a 10-day course of antibiotics, his symptoms persisted. Two months later, further workup included a computed tomography (CT) scan of the sinus which demonstrated extensive bony erosion with significant right maxillary sinus disease (Figure 1).
He underwent right maxillary antrostomy, partial ethmoidectomy and maxillary debridement. Magnetic resonance imaging (MRI) was suggestive of invasive fungal sinusitis extending into the orbit, and preliminary pathology evidenced invasive fungal disease (Figure 2A). The patient was started on amphotericin B lipid (5mg/kg) and transferred to our institution for further management.
On arrival his temperature was 36.8 °C, BP of 148/73 mmHg, HR 72 bpm, RR 18/min, oxygen saturation 99 % at room air. He complained of right facial pain of 10 on a pain scale from 1-10 and intermittent diplopia on the right site after prior intervention. The positive findings on his physical examination were: tenderness to palpation over the right frontal and maxillary sinuses and proptosis of the right eye with full visual acuity 20/25 and 20/30, pupil 3 mm and 2 mm, and intraocular pressure 20 and 17 mmHg on the right and left eye respectively, with external ocular movements, V1 and V2 intact. His initial lab work showed WBC: 5.5 x 103/uL, Hb 10.4 g/dl, Platelets 164 x 103/uL, BUN 17 mg/dl, serum creatinine 0.6 mg/dl and tacrolimus trough level of 3.7 ng/ml.
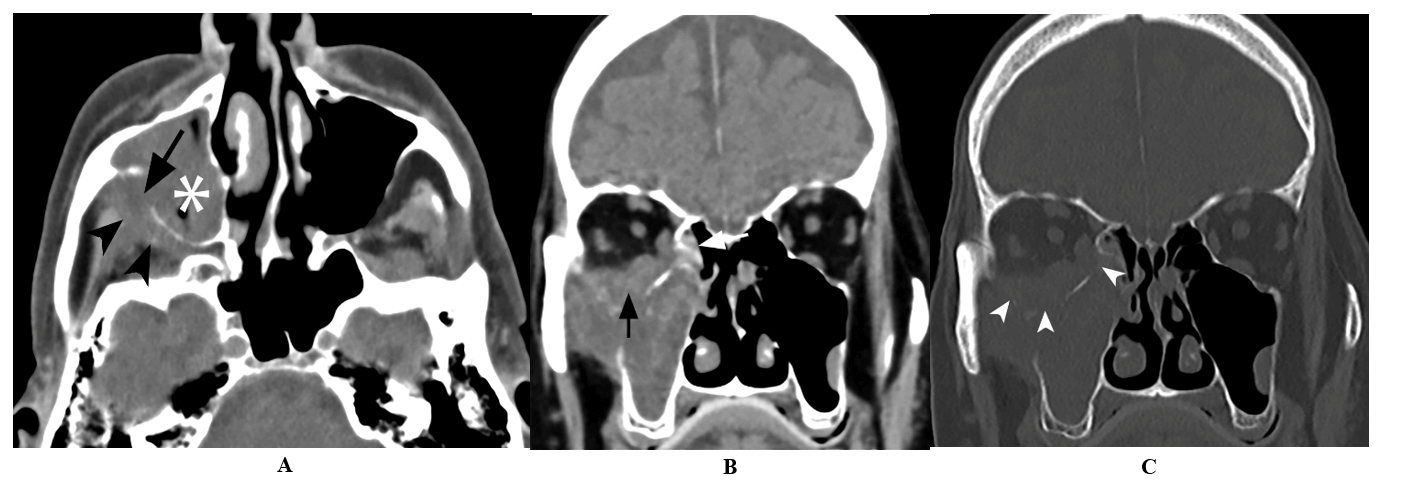
Figure 1: A) Axial unenhanced CT image demonstrate opacification of the right maxillary sinus (asterisk) with destruction of the posterior sinus wall (arrow) and obliteration of the retroantral fat plane (arrowheads). B) & C) Coronal unenhanced CT images (soft tissue and bone windows) demonstrate soft tissue thickening in the right posterior ethmoid air cells (arrow) with destruction of the medial and inferior wall of the orbit (arrowheads) and orbital extension.
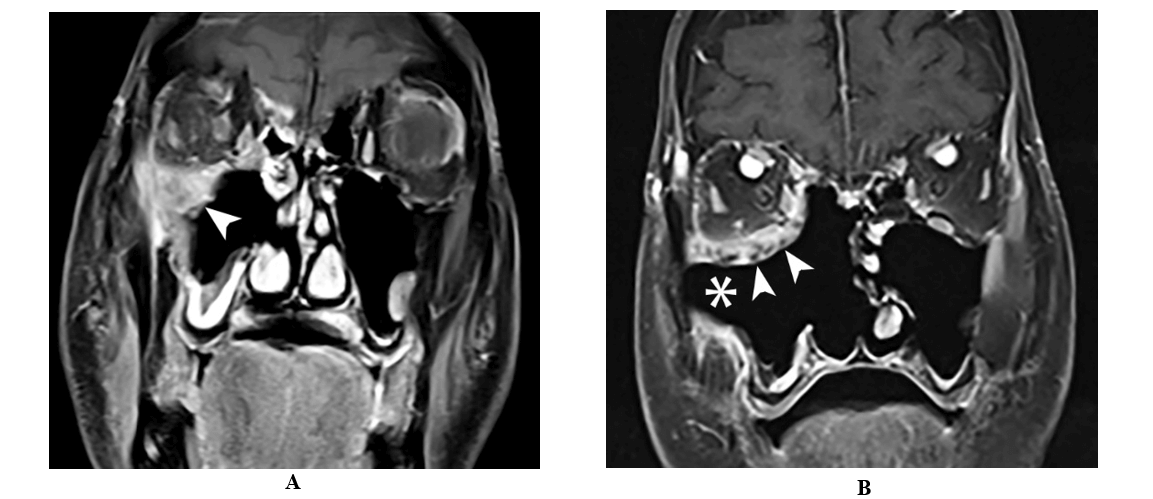
Figure 2: A) Coronal T1 weighted fat saturated contrast enhanced MRI image following right maxillary antrostomy, partial ethmoidectomy and maxillary debridement shows residual enhancing soft tissue in the medial and inferior orbital wall with involvement of the infraorbital canal (arrow). B) Coronal T1 weighted fat saturated contrast enhanced image following extensive sinonasal surgery showing with right medial maxillectomy and total ethmoidectomy with interval debridement of soft tissue in the maxillary cavity (asterisk) and orbital floor (arrow).
He underwent same day endoscopic sinus surgery with right medial maxillectomy, total ethmoidectomy, sphenoidectomy, frontal sinusotomy and trans nasal trans maxillary resection of soft tissue from the infratemporal and pterygopalatine fossa. Frozen section was reported as invasive mycosis with presence of septate hyphae suggesting Aspergillus in the anterior and posterior mid orbital wall. His antifungal therapy was changed from amphotericin B to voriconazole and micafungin. In addition, the immunosuppressive therapy with mycophenolate mofetil was discontinued and his tacrolimus target through levels were maintained at 3-5 ng/ml by reducing the dose of tacrolimus to one-third due to drug-drug interaction attributed to a strong inhibitory effect on CYP3A4 activity by voriconazole.
A CT scan of the chest showed mild emphysema and multiple bilateral sub centimeter nonspecific pulmonary nodules up to 2 mm in size. Orbital and brain MRI showed enhancing soft tissue in the orbital floor involving the lateral, medial and inferior rectus muscles and postsurgical changes without intracranial involvement (Figure 2B). Four days later he underwent further debridement of the pterygoid muscles with resection of the maxillary nerve up to the foramen rotundum. The final surgical cultures confirmed Aspergillus fumigatus. The patient´s condition improved through his hospital course with resolution of diplopia without requiring orbital exenteration and he was discharged on voriconazole monotherapy.
He completed a total of 9 months treatment with voriconazole under the supervision of infectious disease. There was no evidence of recurrence by ENT with direct endoscopic examination; along with Ophthalmology and Neurosurgery follow up which included sequential MRI studies performed at 2 and 8 months after starting voriconazole (Figure 3). Despite lowering immunosuppressive regimen, the patient also developed Cytomegalovirus (CMV) viremia two months after discharge for which he was treated with valganciclovir until complete resolution. His kidney function remained stable with a serum creatinine of 0.5 mg/dl while on maintenance immunosuppression with tacrolimus and prednisone. He sustained complete resolution of initial persistent chronic facial neuropathic pain after one year of the initial presentation.
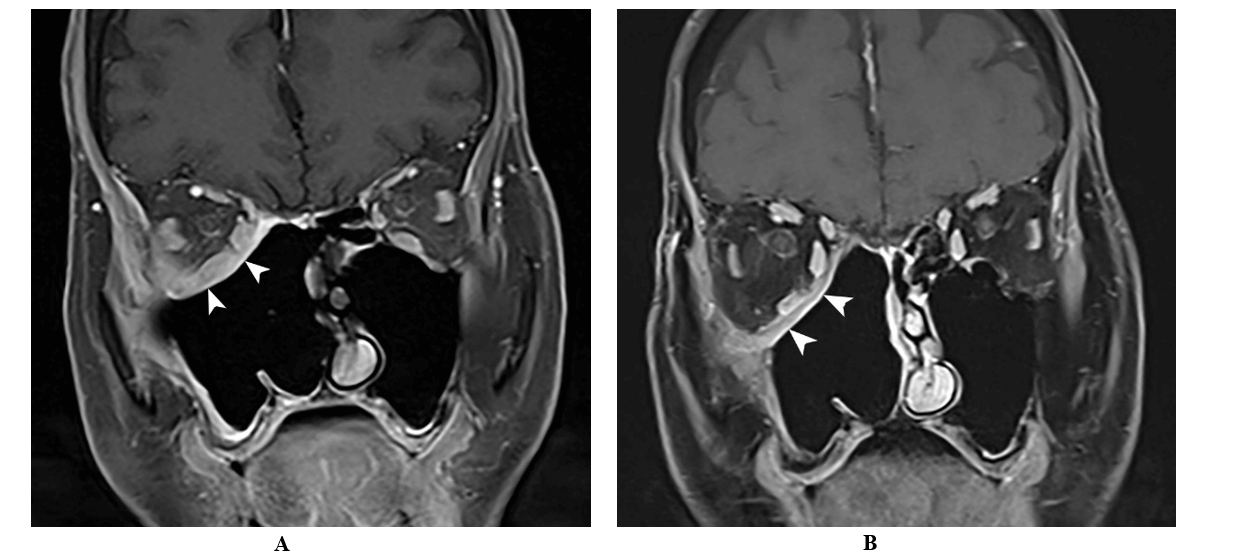
Figure 3: Coronal T1 weighted MRI after A) 2 months and B) 8-months of treatment showed continued interval improvement of the residual enhancing soft tissue within the operative bed (arrow).
Discussion
Solid organ transplant (SOT) recipients require long term use of immunosuppressive medications that place them at risk for opportunistic infections. Aspergillus species are ubiquitous in nature and the inhalation of infectious conidia is a common event that can cause in immunocompromised patients a spectrum of complications ranging from invasive to non-invasive disease [1]. Aspergillosis of the paranasal sinuses is subdivided into noninvasive and invasive types, depending on the depth of involvement of the tissue. The invasive form often occurs in immunodeficient patients and can be divided further into granulomatous, chronic invasive, and acute fulminating forms [2]. Tissue invasion is characterized by progression of the infection across tissue planes. It is uncommon and occurs most frequently in the setting of SOT [3].
Most common Aspergillus infections are caused by A. fumigatus (67%), followed by A. flavus (13%), A. niger (9%) and A. terreus (7%) species as reported from 24 transplant centers in the USA in contrast to data from a decade before that showed that A. fumigatus was exclusively involved in up to 90% of the invasive cases [4]. Typically, invasive aspergillosis involves the lungs, but the infection can disseminate beyond the respiratory tract to multiple different organs, including the skin, brain, eyes, liver and kidneys with a very poor prognosis. Rarer manifestations include tracheobronchitis, rhinosinusitis, brain abscess, endophthalmitis, endocarditis and gastrointestinal disease. Aspergillus endophthalmitis with progressive infection is characterized by the destruction of multiple components of the eye with usually implies vision loss and the need of exenteration in some cases [5, 6].
Initial presentation of invasive aspergillosis is often after the orbit or cranial vault have been invaded. Bony erosion allows the spread of fungal infection to these structures, and is thought to result from increased pressure, demineralization of bone, or expansion of fungal mass [7-9]. Intra-cranial involvement may occur by direct extension through the superior orbital fissure, hematogenous spread, or erosion through the affected sinus [9]. Radiographic features of sino-orbital aspergillosis may include heterogeneous masses within the paranasal sinuses, bony erosions, and heterogeneous masses within the orbit that attenuate with contrast [9, 10].The diagnosis is made by biopsy and culture and often multiple biopsies are required [11].Differential diagnosis of fungal rhinosinusitis includes Mucormycosis and other non-Aspergillus molds [4]. Most recipients of SOT with invasive disease acquire the infection through a high amount of airway exposure as in the setting of being near local construction areas or, especially in lung, heart-lung and liver recipients, if the patients were to experience a prolong period of neutropenia [12, 13].
In renal transplant recipients, invasive aspergillosis has been reported in 0.7-4% [14, 15]. Traditional risk factors for this population are the use of high doses of methylprednisolone (> 3 grams) or prolonged use of corticosteroids, and graft failure requiring dialysis [14, 16, 17]. In a recent multinational case-control study, new risk factors were identified for early-onset infection (≤3 months following transplantation) which include longer duration of hemodialysis before transplantation, the diagnosis of chronic obstructive pulmonary disease, delayed graft function, bacteremia and leukopenia [18]. CMV disease, pneumonia, tuberculosis or other complications associated with over immunosuppression (e.g. post-transplant lymphoprolipherative disease were identified as independent risk factors for late-onset infection (>3 months following transplantation) [14, 16, 17]. The use of amphotericin B, caspofungin and voriconazole has been described in the literature to successfully treat this frequently fatal condition [19, 20]. This is the third case of sino-orbital invasive aspergillosis reported in kidney transplant recipients and the first to achieve resolution on voriconazole monotherapy [20].
Conclusion
This case illustrates a rare but life-threatening condition in kidney transplant recipients called sino-orbital aspergillosis that highlights the importance of suspecting and promptly recognizing the presence of fungal infection in the paranasal sinuses in order to avoid significant morbidity and mortality. In the treatment of culture-proven orbital aspergillosis, the use of voriconazole as first-line medical therapy should now be considered.
Conflicts of Interest
None.
Author Contributions
All authors contributed to all aspects of the article.
Abbreviations
CMV: cytomegalovirus
CT: computed tomography
ENT: ears, nose and throat
MRI: magnetic resonance imaging
SOT: solid organ transplant
V1: cranial nerve V: test sensation above eyes
V2: cranial nerve V: test sensation below eyes and above mouth
Article Info
Article Type
Case ReportPublication history
Received: Thu 19, Mar 2020Accepted: Thu 02, Apr 2020
Published: Mon 13, Apr 2020
Copyright
© 2023 Adela D. Mattiazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.TCR.2020.01.04
Author Info
Adela D. Mattiazzi Camilo A. Cortesi Efrat Saraf Lavi Giselle Guerra Jose F. Camargo Warren L. Kupin
Corresponding Author
Adela D. MattiazziDepartment of Medicine, Division of Nephrology, University of Miami Miller School of Medicine, Miami, Florida, USA
Figures & Tables
References
- Segal BH (2009) Aspergillosis. N Eng J Med 360: 1870-1884. [Crossref]
- Götze G, Bloching M, Hainz M, Knipping S (2007) Invasive aspergillosis of the skull base with orbit infiltration. HNO 55: 560-563. [Crossref]
- Ben Ami R, Lewis RE, Kontoyiannis DP (2010) Enemy of the (immunosuppressed) state: and update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol 150: 406-417. [Crossref]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L (200l) Epidemiology and outcome of mold infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 34: 909-917. [Crossref]
- Levin LA, Avery R, Shore JW, Woog JJ, Baker AS (1996) The spectrum of orbital aspergillosis: a clinicopathological review. Surg Ophtalmol 41: 142-154. [Crossref]
- Ridell I, McNeil SA, Johnson TM, Bradley SF, Kazanjian PH et al. (2002) Endogenous Aspergillus endophthalmitis: report of 3 cases and review of literature. Medicine (Baltimore) 81: 311-320. [Crossref]
- Halliday L, Curragh D, Selva D (2019) A rare case of invasive sino-orbital aspergillosis arising from isolated frontal sinus infection. Can J Ophtalmol 54: e19-e21. [Crossref]
- Gupta R, Gupta AK (2013) Isolated primary frontal sinus aspergillosis: role of endonasal endoscopic approach. J Laryngol Otol 127: 274-278. [Crossref]
- Alaraj AM, Al Faky YH, Alsuhaibani AH (2018) Ophthalmic manifestations of allergic fungal sinusitis. Ophthal Plast Reconstr Surg 34: 463-466. [Crossref]
- Sivak Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P et al. (2004) Localised invasive sino-orbital aspergillosis: characteristic features. Br J Ophthalmol 88: 681-687. [Crossref]
- Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P et al. (2006) Comparison of epidemiological, clinical and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients; a 6-year survey. Clin Infect Dis 43: 577-584. [Crossref]
- Kanamori H, Rutala WA, Sickbert Bennett EE, Weber DJ (2015) Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 61: 433-444. [Crossref]
- Paterson DL, Singh N (1999) Invasive aspergillosis in transplant recipients. Medicine (Baltimore) 78: 123-138. [Crossref]
- Altiparmak MR, Apaydin S, Trablus S, Serdengecti K, Ataman R et al. (2002) Systemic fungal infections after renal transplantation. Scand J Infect Dis 34: 284-288. [Crossref]
- Gustafson TL, Schaffner W, Lavely GB, Stratton CW, Johnson HK et al. (1983) Invasive aspergillosis in renal transplant recipients: correlation with corticosteroid therapy. J Infect Dis 148: 230-238. [Crossref]
- Panackal AA, Dahlman A, Keil KT, Peterson CL, Mascola L et al. (2003) Outbreak of invasive aspergillosis among renal transplant recipients. Transplantation 75: 1050-1053. [Crossref]
- Lopez Medrano F, Fernandez Ruiz M, Silva JT, Carver PL, van Delden C et al. (2018) Multinational case-control study of risk factors for the development of late invasive pulmonary aspergillosis following kidney transplantation. Clin Microbiol Infect 24: 192-198. [Crossref]
- Singh N, Paterson DL (2005) Aspergillus infections in transplant recipients. Clin Microbiol Rev 18: 44-69. [Crossref]
- Heylen L, Maertens J, Naesens M, Van Wijngaerden E, Lagrou K et al. (2015) Invasive aspergillosis after kidney transplant: case-control study. Clin Infect Dis 60: 1505-1511. [Crossref]
- Said T, Nampoory MR, Nair MP, Al Saleh M, Al Haj KH et al. (2005) Safety of caspofungin for treating invasive nasal sinus aspergillosis in a kidney transplant recipient. Transplant Proc 37: 3038-3040. [Crossref]
