A Preliminary Study of the Absorption and Metabolism of Temperate Propolis by Human Subjects
A B S T R A C T
Temperate propolis is collected by bees from the sticky secretions produced by the buds of poplar species. It is used to coat and seal the hive thus protecting against infections. Extensive research indicates that propolis has strong anti-protozoal activity. There have been some large clinical trials testing propolis against a variety of conditions but despite there is no information on whether or not the active components in propolis are absorbed by humans. In order to answer this question, a small study was carried out in order to determine whether or not propolis components could be detected in the urine of 5 human subjects taking a small dose of propolis tincture. In two of the subjects, levels of several of the flavonoids present in the propolis tincture many times above the baseline levels were detected following hydrolysis of the urine samples with a glucuronidase/sulfatase. Analysis of urine samples prior to hydrolysis indicated the presence of glucuronides and sulfates of the main flavonoids in propolis. Flavonoid absorption occurred to some degree in all subjects, apparent lower levels of flavonoid absorption in 3 out of five subjects might indicate genuine differences in level of absorption between subjects.
Keywords
Propolis, absorption and metabolism, humans, high resolution mass spectrometry
Introduction
Propolis is a resinous substance collected by bees, generally from plant buds. Its composition varies widely according to the vegetation surrounding the beehive [1]. It is collected on the hindlegs of the bee and is removed with the help of other bees upon return to the hive and layered onto surfaces and used to fill any gaps within the hive, helping to maintain a sterile environment within the hive. In Northern Europe and other temperate regions such as Northern China and North America propolis is generally collected from the buds of poplar species, whereas in Southern Europe the predominant sources are various Cypress species and in tropical regions several different plant sources may be utilized [1]. Propolis is widely available as a food supplement and a wide range of biological properties have been attributed to it including immunomodulatory, anti-diabetic, anti-microbial and anti-cancer activity [1, 2]. In temperate propolis, which is largely collected from poplar species, and provides the basis for most of the commercially available propolis preparations, the major components are flavonoids and metabolites of caffeic acid [1, 3, 4]. In addition, temperate propolis has potentially important biological activity in having strong activity against protozoa, particularly Leishmania [5-9]. All in vitro evidence so far points towards this activity being due to flavonoids. Given the low toxicity of propolis, high doses of propolis preparations could be given and it might prove to be an effective treatment, particularly for Leishmaniasis which is still widespread in some areas of the World and is currently treated by chemotherapy with high levels of toxicity to the host [10].
The have been quite a number of large-scale trials on patients using propolis to treat various conditions with few adverse events being reported [11-21]. Some previous studies using propolis in a clinical setting are summarized below. One concern is potential allergic reactions; however, reports of allergic reactions are rare. A survey of Polish beekeepers and their families, taking propolis, reported allergy to propolis in 14 out of 2205 people surveyed [22]. Thus, the toxicity of propolis appears to be low. Most of these trials are summarized below:
The effect of propolis on insulin resistance and lipid profile was assessed. 50 patients in the treated group were give 1000 mg of propolis daily for 90 days. No adverse events were reported for the treatment group [11]. The efficacy of propolis in treating respiratory infection in children was tested. A preparation containing propolis at was administered at up to a dosage of 550 mg up to 4 times daily to children aged 4-5 years over 5 months. 328 children completed the study with 99 dropping out, the most common reason for withdrawal was the unpleasant taste of the preparation. No adverse events were reported [12]. Propolis/zinc suspension was tested for the prevention of acute otitis with propolis at a level of 100 mg/kg per day in children mean aged >30 months. The trial was carried out on 115 children and continued for a month. Two adverse events were reported in the treated group [13].
The efficacy of propolis in the treatment of type II diabetes was assessed. 31 patients in the treatment group took 900 mg of propolis daily for 18 weeks. 25 completed in trial with four withdrawing because of allergic reaction [14]. Propolis was assessed as a treatment for infertility associated with mild endometriosis; 20 patients in the treatment group took 1000 mg of propolis daily for 9 months. No adverse events were recorded [15]. The effectiveness of propolis as prophylactic immune-stimulating agent was assessed in 10 healthy subjects who received 500 mg of propolis per day over 13 days. No side effects were reported [16].
Propolis was tested for reducing oxidative stress in smokers. 29 smokers received 800 mg of propolis per day for 4 weeks [17]. No adverse events were reported. In a trial testing the efficacy of propolis in treating type II diabetes, 31 patients in the treatment group were given 1500 mg of propolis per day for 8 weeks. 30 out of 31 patients completed the study with one being excluded due to changes in their antidiabetic medicine [18]. Propolis was tested as a potential treatment of ulcerative oral lichen planus. 27 patients were given 500 mg of propolis daily over 30 days. During the course of the study two patients were withdrawn from the study [19]. In an experimental treatment of uterine myoma 500 mg of propolis was given three times daily to 20 patients over 12 weeks. No adverse events were reported [20]. In a study of the treatment of dysmenorrhea 500 mg of propolis per day was given to 86 subjects over two months, no adverse events were reported [21].
It is clear from these relatively large trials that propolis is well tolerated in human subjects, but the question remains as to whether or not the major components in propolis are absorbed. Given the low toxicity of propolis, high doses of propolis preparations could be given and it might prove to be an effective treatment, particularly for Leishmaniasis. The collection of propolis bees with anti-protozoal activity increasingly appears to be deliberate. The Scottish honeybee microbiome contains high levels of genetic material derived from the trypanosomatid Lotmaria passim and this organism has been found to be widespread in other bee populations [23-26]. The spread of the protozoal infection in bees may occur via faeces and coating the surfaces in the hive with propolis that is active against trypanosomatids could prevent transmission [27]. Recently it has been found that feeding propolis to bees reduces Nosema ceranae infection [28].
Thus, the following paper proposes to answer the question, in a qualitative manner, with regard to whether or not the major components of propolis are absorbed from an oral dose and to also determine the nature of any metabolites produced. Propolis, whatever its origin, always exhibits high levels of anti-protozoal activity. The highest activity is generally associated with flavonoids.
Materials and Methods
I Chemicals
HPLC grade acetonitrile, formic acid and water were obtained from Fisher Scientific (Leicestershire UK), Helix pomatia sulfatase-glucuronidase type H2 was obtained from Sigma Aldrich (Dorset, UK). The flavonoid standards were obtained from obtained from Sigma Aldrich (Dorset, UK).
II Samples
The small trial was approved by the University of Strathclyde ethics committee (UEC19/49: Watson: Determination of the urinary metabolites of propolis). The propolis sample used was a 50% solution of propolis in glycerol from Y.S. Eco Bee Farms (Sheridan, IL, USA). Participants were instructed to take 15 drops of propolis tincture mixed with a teaspoon full of honey in two doses (equivalent to ca 300 mg of propolis per dose) and collect four samples of urine in accordance with the diagram shown in (Figure 1). For analysis of flavonoid metabolites 0.5 ml of urine was mixed with 0.5 ml of acetonitrile, the sample was centrifuged at 3000g and the supernatant was then injected into the LC-MS. For analysis of the unconjugated flavonoids, 0.5 ml of urine was mixed with 0.1 ml of 1 M sodium acetate buffer pH 5.0 and 20 µl of Helix pomatia sulfatase-glucuronidase type H2 (Sigma Aldrich, Dorset, UK) and incubated at 37°C for 1 h. The solution was then mixed with 0.6 ml of acetonitrile and the supernatant was then injected into the LC-MS.
Figure 1: Protocol for dosing of the propolis extract.
III LC-MS Analysis
LC-MS was carried out by using an Accela pump connected to an Orbitrap Exactive mass spectrometer operated in positive/negative switching mode. The sheath gas and auxiliary gas were set at 50 and 17 arbitrary units, respectively. The needle voltage was 4.5 kV in positive mode and 4.0 kV in negative mode. The heated capillary temperature was 320 °C. The HPLC was fitted with an ACE C18 column 150 × 4.6 mm, 3 µM particle size (Hichrom, Reading, UK). Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in acetonitrile. The flow rate was 0.3 ml/min and the solvent gradient was as follows: 0 min 10% B, 30 min 90 % 30 min 30% B, 31 min 10% B, 36min. The files were processed by using m/z Mine 20.1 and then the extracted masses were searched against an in-house database.
Results and Discussion
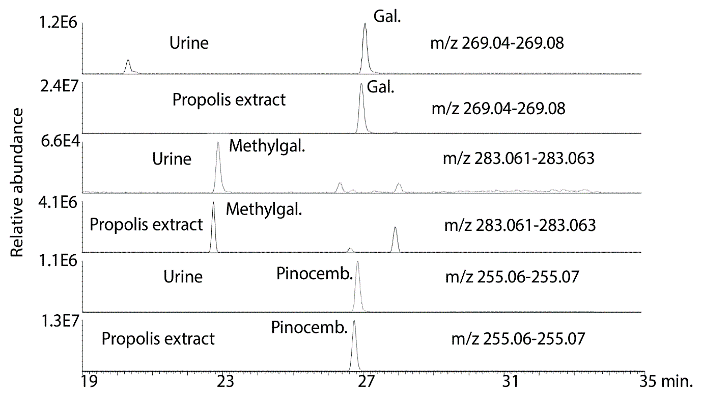
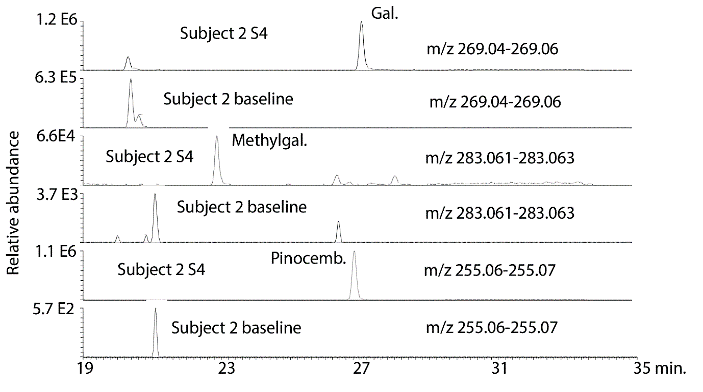
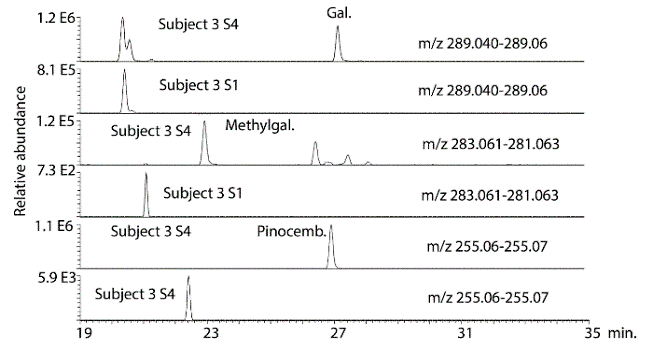
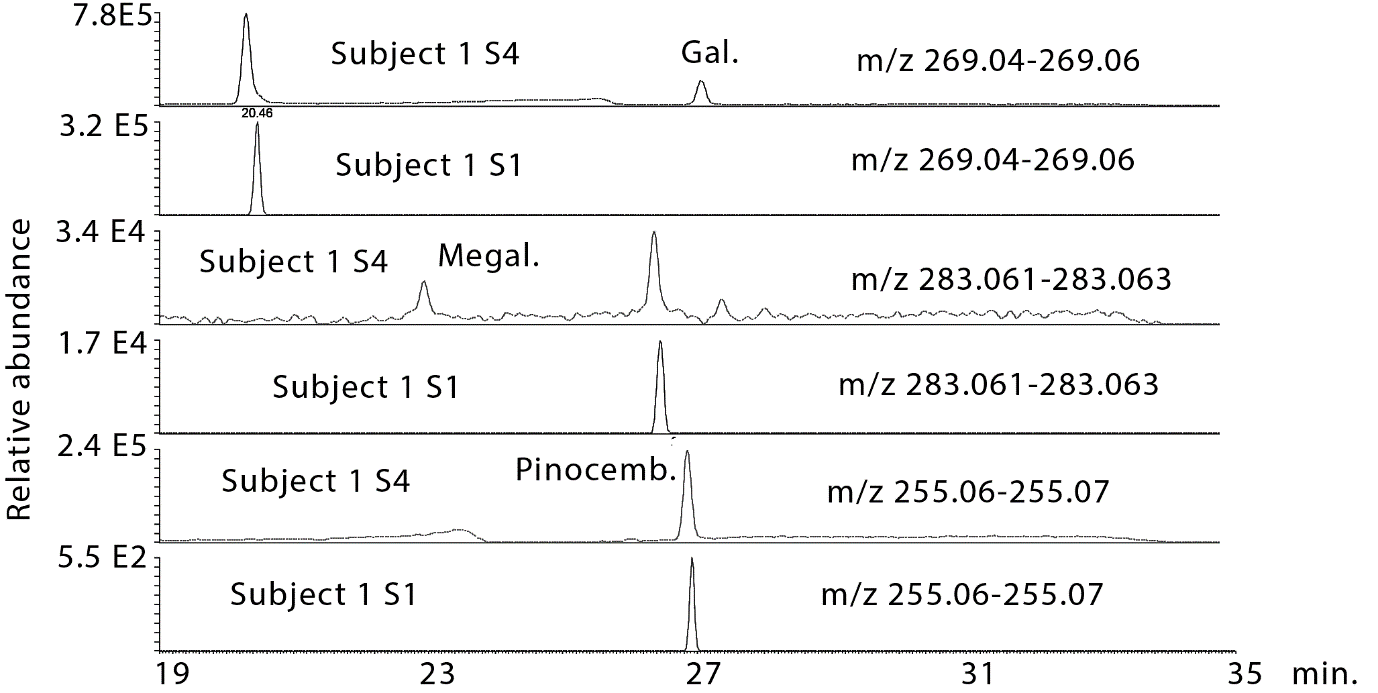
There was clear evidence of absorption of the major flavonoids in all of the subjects although this appeared to be variable which is perhaps not surprising since the protocol for dosage and collection were only loosely controlled. Figure 2 shows galangin, methyl galangin and pinocembrin, which are major components in propolis, in the propolis extract taken by the subjects in comparison with galangin, methyl galangin and pinocembrin in the urine sample 4 from subject 2 following hydrolysis. Figure 3 shows the same compounds in sample 4 from subject 2 in comparison with sample 1, the baseline sample, from subject 2. There is no galangin, methyl galangin or pinocembrin detectable in the baseline sample.
Figure 2: Extracted ion traces for some flavonoids in hydrolysed urine sample 4 from subject 2 in comparison with flavonoids in the propolis tincture taken by the subjects.
Figure 3: Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 2 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 1.
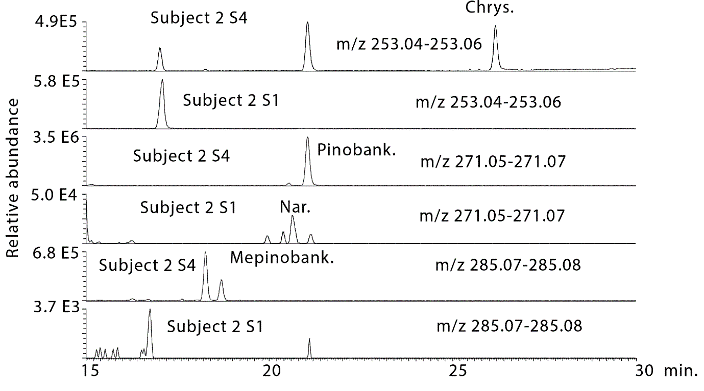
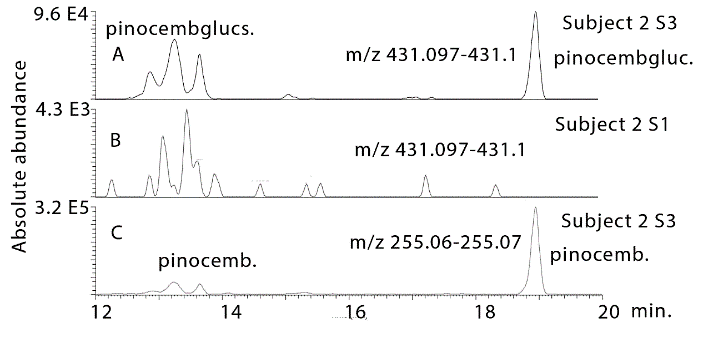
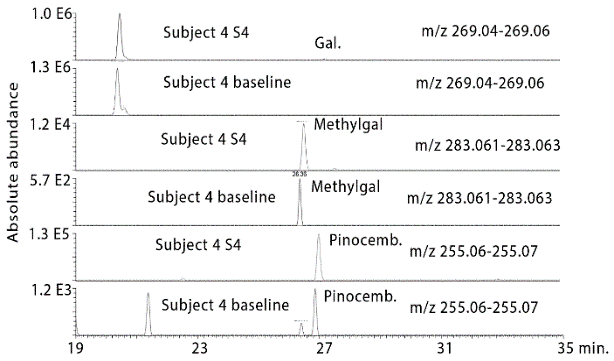
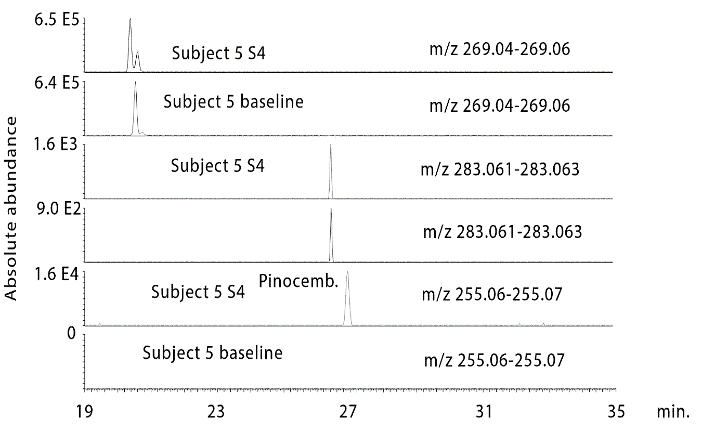
Figure 4 shows extracted ion traces for chrysin in urine sample 4 from subject 2 where two isomers are present at many times above baseline. In addition, pinobanksin is present at many times the baseline level in the sample as are two isomers of methyl pinobanksin. There is an isomer of pinobanksin present in the sample at a retention time corresponding to that of narigenin which is present in orange juice. In addition, there appear to small amounts of two isomers of methyl galangin present in the baseline sample. The picture is similar if the sample 4 and the baseline sample for subject 3 are examined (Figure S1). Comparing sample 1 and sample 4 for subject 4 indicates clear absorption of the three marker compounds, although less efficient absorption than for subjects 2 and 3. Table 1 shows the retention times for some standards for the compounds found in propolis. Table 2 summarizes the ratios for the flavonoids which can be observed in the propolis extract in the three post-dose samples in comparison to the baseline samples. The average levels observed post-dose are many times those observed in the baseline samples. However, there are wide variations in the levels obtained in each of the five subjects (Figures S2-S4) but by taking the log10 values for the peak areas the data skewing is reduced sufficiently to obtain significant P values for most of the flavonoids.
Figure 4: Extracted ion traces for chrysin (chrys), pinobansksin (pinobank) methylpinobanksin (methylpinobank) in hydrolysed urine sample 4 from subject 2 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 2.
Table 1: Retention times and masses for flavonoid standards run on an ACE C18 column.
|
Compound |
Rt (min) |
m/z |
|
Chrysin |
26.2 |
253.0506 |
|
Pinocembrin |
26.8 |
255.0663 |
|
Apigenin |
20.4 |
269.0457 |
|
Galangin |
27.1 |
269.0457 |
|
Narigenin |
20.5 |
271.0614 |
|
Pinobanksin |
21.2 |
271.0614 |
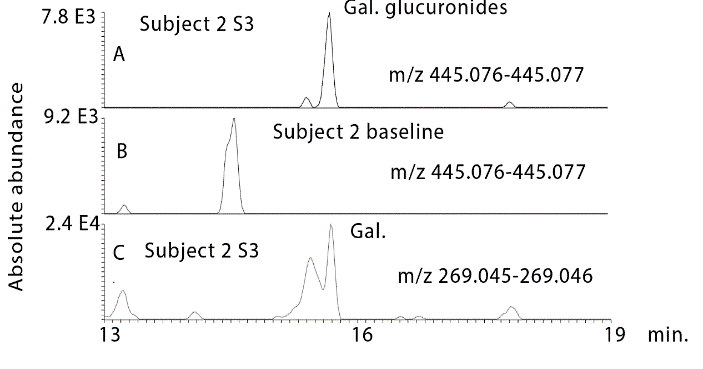
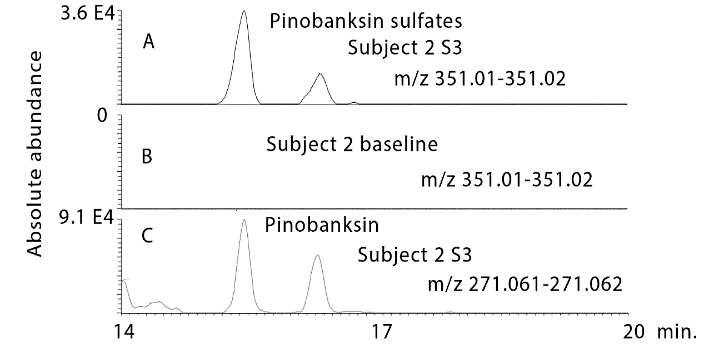
Prior to hydrolysis several glucuronides of the flavanones could be observed in the urine samples. The intensity of the peaks was reduced in comparison to the unconjugated flavanones since each flavanone produced several glucuronide metabolites. Figure 5A shows the most abundant glucuronide which is for pinocembrin in subject 2 sample 3, (Figure 5B) shows that this peak is absent in the baseline sample for subject 2 and (Figure 5C) shows an extracted ion trace for in source neutral loss fragmentation generated from the glucuronides producing ions corresponding to the MW of pinocembrin. At least 3 glucuronides can be observed in addition to the peak at 18.9 min. Figure 6 shows a similar trace for glucuronides of galangin where two major peaks are observed. In addition, sulphates were formed, these were observed in urine most clearly for pinocembrin (Figure 7). Despite propolis having been widely used for many years as a treatment for a wide range of conditions there is no information with regard to the absorption of the major components in the mixture. The absorption of dietary flavonoids has been widely studied but as can be seen from the baseline profiles of the urine samples in the current study there is no evidence for high baseline levels of the flavonoids found in propolis and there is no prior assessment of the absorption of this particular group of flavonoids [29].
Table 2: Ratios of flavonoid compounds present in urine at three time points in comparison with baseline levels and P values (n=5).
|
Compound |
Rt min |
P value S1S2 |
Ratio S2/S1 |
P value S1S3 |
Ratio S3/S1 |
P value S1S4 |
Ratio S4/S1 |
|
Chrysin isomer |
21.2 |
0.344 |
52 |
<0.001 |
452 |
0.001 |
>1000 |
|
Chrysin |
26.3 |
0.017 |
296 |
0.016 |
>1000 |
<0.001 |
>1000 |
|
Pinocembrin |
26.9 |
0.003 |
28 |
0.004 |
43 |
0.038 |
122 |
|
Pinocembrin isomer |
13.6 |
0.273 |
28 |
0.002 |
43 |
0.141 |
122 |
|
Pinocembrin isomer |
13.1 |
0.178 |
29 |
0.028 |
43 |
0.297 |
422 |
|
Pinocembrin isomer |
17.8 |
0.981 |
4 |
0.008 |
15 |
0.125 |
20 |
|
Galangin |
27.1 |
0.412 |
10 |
0.075 |
5 |
0.029 |
>1000 |
|
Pinobanksin |
21.2 |
0.050 |
18 |
0.012 |
14 |
0.016 |
64 |
|
Pinobanksin isomer |
15.3 |
0.147 |
111 |
0.76 |
12 |
0.042 |
197 |
|
Methylgalangin isomer |
22.9 |
0.352 |
1 |
0.020 |
12 |
0.098 |
43 |
|
Methylgalangin isomer |
18.6 |
0.374 |
0 |
<0.001 |
2 |
0.012 |
45 |
|
Methylgalangin isomer |
26.4 |
0.135 |
5 |
0.027 |
5 |
0.040 |
19 |
|
Methylpinobanksin isomer |
18.3 |
0.398 |
105 |
<0.001 |
5 |
0.009 |
18 |
Figure 5: Extracted ion traces for A) pinocembrin glucuronides in urine sample 3 from subject 2 in comparison with B) the baseline sample for subject 2. C) Source fragments generated the mass of pinocembrin via a neutral loss.
Figure 6: Extracted ion traces for A) galangin glucuronides in urine sample 3 from subject 2 in comparison with B) the baseline sample for subject 2. C) Source fragments generated the mass of galangin via a neutral loss of glucuronide.
The major components in propolis are flavonoids and plus several esters of these compounds, particularly esters of pinobanksin, and altogether there are several hundred components in temperate propolis with around 20 components being abundant [1, 4]. As might be expected, since esters are rapidly metabolized by the body, although peaks for several esters of pinobanksin were observed in the propolis tincture itself these were not observed in the urine samples probably due to their rapid conversion to pinobanksin by esterases in the body. The extent of absorption of the flavonoids was very variable between the five subjects in the study and a much stricter control of dosing and sample collection would be required to assess the true variability of absorption between subjects. In two of the subjects, absorption of the flavonoids was very efficient with levels of the major flavonoids being many times above the baseline level.
Figure 7: Extracted ion traces for A) pinobanksin sulphates in a urine sample 3 from subject 2 in comparison with B) the baseline sample for subject 2. C) Source fragments generated the mass of pinobanksin via a neutral loss of glucuronide.
Although the current study is somewhat limited in its scope it is now possible to state that many of the major components of propolis can be absorbed by the body and thus could exert a therapeutic effect. This is of particular interest regarding the potential activity of propolis against Leishmaniasis where recently we were able to associate strong activity in vivo against Leishmania with several of the flavonoids in temperate propolis [9]. The established low toxicity of propolis means that high doses could be given in order to treat this infection. There is now clear evidence that the major bioactive metabolites in temperate propolis can be absorbed by the human body. This is information that was not available previously and is an important observation for supporting the medicinal claims made for propolis products.
Supplementary Materials
Figure S1 Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 3 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 3. Figure S2 Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 4 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 4. Figure S3 Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 1 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 1. Figure S4 Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 5 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 5.
Figure S1: Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 3 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 3.
Figure S2: Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 4 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 4.
Figure S3: Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 1 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 1.
Figure S4: Extracted ion traces for some galangin (gal), methylgalangin (methylgal) and pinocembrin (pinocemb) in hydrolysed urine sample 4 from subject 5 in comparison with the same flavonoids in the hydrolysed baseline urine sample from subject 5.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 04, May 2020Accepted: Mon 18, May 2020
Published: Wed 27, May 2020
Copyright
© 2023 Dave Watson. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2020.01.06
Author Info
Abdullah Alotaibi Abdulmalik Alqarni Adel Alghamdi Dave Watson James Fearnley Mohammed Al Rofaidi
Corresponding Author
Dave WatsonStrathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, UK
Figures & Tables
Table 1: Retention times and masses for flavonoid standards run on an ACE C18 column.
|
Compound |
Rt (min) |
m/z |
|
Chrysin |
26.2 |
253.0506 |
|
Pinocembrin |
26.8 |
255.0663 |
|
Apigenin |
20.4 |
269.0457 |
|
Galangin |
27.1 |
269.0457 |
|
Narigenin |
20.5 |
271.0614 |
|
Pinobanksin |
21.2 |
271.0614 |
Table 2: Ratios of flavonoid compounds present in urine at three time points in comparison with baseline levels and P values (n=5).
|
Compound |
Rt min |
P value S1S2 |
Ratio S2/S1 |
P value S1S3 |
Ratio S3/S1 |
P value S1S4 |
Ratio S4/S1 |
|
Chrysin isomer |
21.2 |
0.344 |
52 |
<0.001 |
452 |
0.001 |
>1000 |
|
Chrysin |
26.3 |
0.017 |
296 |
0.016 |
>1000 |
<0.001 |
>1000 |
|
Pinocembrin |
26.9 |
0.003 |
28 |
0.004 |
43 |
0.038 |
122 |
|
Pinocembrin isomer |
13.6 |
0.273 |
28 |
0.002 |
43 |
0.141 |
122 |
|
Pinocembrin isomer |
13.1 |
0.178 |
29 |
0.028 |
43 |
0.297 |
422 |
|
Pinocembrin isomer |
17.8 |
0.981 |
4 |
0.008 |
15 |
0.125 |
20 |
|
Galangin |
27.1 |
0.412 |
10 |
0.075 |
5 |
0.029 |
>1000 |
|
Pinobanksin |
21.2 |
0.050 |
18 |
0.012 |
14 |
0.016 |
64 |
|
Pinobanksin isomer |
15.3 |
0.147 |
111 |
0.76 |
12 |
0.042 |
197 |
|
Methylgalangin isomer |
22.9 |
0.352 |
1 |
0.020 |
12 |
0.098 |
43 |
|
Methylgalangin isomer |
18.6 |
0.374 |
0 |
<0.001 |
2 |
0.012 |
45 |
|
Methylgalangin isomer |
26.4 |
0.135 |
5 |
0.027 |
5 |
0.040 |
19 |
|
Methylpinobanksin isomer |
18.3 |
0.398 |
105 |
<0.001 |
5 |
0.009 |
18 |
References
- Bankova V, Popova M, Trusheva B (2018) The phytochemistry of the honeybee. Phytochemistry 155: 1-11. [Crossref]
- Alqarni AM, Niwasabutra K, Sahlan M, Fearnley J, Fearnley H et al. (2019) Propolis Exerts an Anti-Inflammatory Effect on PMA-Differentiated THP-1 Cells via Inhibition of Purine Nucleoside Phosphorylase. Metabolites 9: E75. [Crossref]
- Wilson MB, Spivak M, Hegeman AD, Rendahl A, Cohen JD (2013) Metabolomics reveals the origins of antimicrobial plant resins collected by honey bees. PloS One 8: e77512. [Crossref]
- Saleh K, Zhang T, Fearnley J, Watson DG (2015) A comparison of the constituents of propolis from different regions of the United Kingdom by liquid chromatography-high resolution mass spectrometry using a metabolomics approach. Curr Metabol 3: 42.
- Siheri W, Alenezi, S, Tusiimire J, Watson DG (2017) The chemical and biological properties of propolis Bee Products - Chemical and Biological 16: 137-178. [Crossref]
- Siheri W, Zhang T, Ebiloma GU, Biddau M, Woods N et al. (2016) Chemical and Antimicrobial Profiling of Propolis from Different Regions within Libya. PLoS One 11: e0155355. [Crossref]
- Siheri W, Ebiloma GU, Igoli JO, Gray AI, Biddau M et al. (2019) Isolation of a Novel Flavanonol and an Alkylresorcinol with Highly Potent Anti-Trypanosomal Activity from Libyan propolis. Molecules 24: E1041. [Crossref]
- Omar RM, Igoli J, Gray AI, Ebiloma GU, Clements C et al. (2016) Chemical characterisation of Nigerian red propolis and its biological activity against trypanosoma brucei. Phytochem Anal 27: 107-115. [Crossref]
- Alotaibi A, Ebiloma GU, Williams R, Alenezi SD, Donachie AM et al. (2019) Sci Rep 9:1-10.
- Torres Guerrero E, Quintanilla Cedillo MR, Ruiz Esmenjaud J, Arenas R (2017) Leishmaniasis: a review. F1000Res 6: 750. [Crossref]
- Zakerkish M, Jenabi M, Zaeemzadeh N Hemmati AA, Neisi N (2019) The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci Rep 9: 7289. [Crossref]
- Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y (2004) Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin c in preventing respiratory tract infections in children: A randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med 158: 217-221. [Crossref]
- Marchisio P, Esposito S, Bianchini S, Desantis C, Galeone C et al. (2010) Effectiveness of a propolis and zinc solution in preventing acute otitis media in children with a history of recurrent acute otitis media. Int J Immunopathol Pharmacol 23: 567-575. [Crossref]
- Gao W, Pu L, Wei J, Yao Z, Wang Y et al. (2018) Serum Antioxidant Parameters are Significantly Increased in Patients with Type 2 Diabetes Mellitus after Consumption of Chinese Propolis: A Randomized Controlled Trial Based on Fasting Serum Glucose Level. Diabetes Ther 9: 101-111. [Crossref]
- Ali AF, Awadallah A (2003) Bee propolis versus placebo in the treatment of infertility associated with minimal or mild endometriosis: A pilot randomized controlled trial. A modern trend. Fertility Sterility 80: 32.
- Brätter C, Tregel M, Liebenthal C, Volk HD (1999) Prophylactic effectiveness of propolis for immunostimulation: a clinical pilot study. Forsch Komplementarmed 6: 256-260. [Crossref]
- Kang MH, Lee HJ, Kim MK, Sung MK, Kwon O et al. (2009) Changes in lymphocyte DNA damage and antioxidant status after supplementing propolis to Korean smokers: A placebo-controlled, double-blind cross-over trial. Korean J Nutr 42: 442-452.
- Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Haghighian HK (2019) Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement Ther Med 43: 283-288. [Crossref]
- Zonouz AT, Mehdipour M, Abadi RTA, Shokri J, Rajaee M et al. (2015) Effect of the use of propolis on serum levels of interleukin-17 and clinical symptoms and signs in patients with ulcerative oral lichen planus. Adv Nat Appl Sci 9: 39-45.
- Ali AFM, Farid L, Shaker S (2018) Bee propolis treatment of uterine myoma a new modality. Life Sci J 15.
- Jenabi E, Fereidooni B, Karami M, Masoumi SZ, Safari M et al. (2019) The effect of bee propolis on primary dysmenorrhea: a randomized clinical trial. Obstet Gynecol Sci 62: 352-356. [Crossref]
- Basista KM, Filipek B (2012) Allergy to propolis in polish beekeepers. Adv Dermatology Allergology/Postepy Dermatologii Alergologii 29.
- Regan T, Barnett MW, Laetsch DR, Bush SJ, Wragg D et al. (2018) Characterisation of the British honey bee metagenome. Nat Commun 9: 4995. [Crossref]
- Schwarz RS, Bauchan GR, Murphy CA, Ravoet J, de Graaf DC et al. (2015) Characterization of Two Species of Trypanosomatidae from the Honey Bee Apis mellifera: Crithidia mellificae Langridge and McGhee, and Lotmaria passim n. gen., n. sp. J Eukaryot Microbiol 62: 567-583. [Crossref]
- Ravoet J, Schwarz RS, Yanez O, Tozkar CO, Martin Hernandez R et al. (2015) Differential diagnosis of the honey bee trypanosomatids Crithidia mellificae and Lotmaria passim. J Invertebr Pathol 130: 21-27. [Crossref]
- Castelli L, Branchichela B, Invernizzi C, Tomasco I, Basualdo M et al. (2018) Detection of Lotmaria passim in Africanized and European honey bees from Uruguay, Argentina and Chile. J Invertebr Pathol 160: 95-97. [Crossref]
- Ruiz Gonzalez MX, Brown MJ (2006) Honey bee and bumblebee trypanosomatids: specificity and potential for transmission. Ecolog Entomol 31: 616-622.
- Mura A, Pusceddu M, Theodorou P, Angioni A, Floris I et al. (2020) Propolis Consumption Reduces Nosema ceranae Infection of European Honey Bees (Apis mellifera). Insects 11: E124. [Crossref]
- Bagwe Parab S, Kaur G, Buttar HS, Singh TH (2019) Absorption, Metabolism, and Disposition of Flavonoids and Their Role in the Prevention of Distinctive Cancer Types. In: Singh Tuli H. (eds) Current Aspects of Flavonoids: Their Role in Cancer Treatment. Springer Singapore.
