A Doctor’s Dilemma: Postoperative Atrial Fibrillation – Should We Anticoagulate?
A B S T R A C T
Background: Postoperative atrial fibrillation (POAF) is a relatively common phenomenon, occurring in approximately 20-40% of cases. Previous studies and guidelines from the AHA/ACC recommended initiating anticoagulation in patients with POAF lasting over 48 hours. However, a few recent studies suggest improved outcomes after anticoagulation even at 5 minutes of POAF. Our meta-analysis aims to clarify primary outcomes of ischemic stroke and mortality in patients with POAF and to offer guidance on whether these patients would benefit from chronic anticoagulation.
Objective: To assess whether patients with POAF benefit from chronic anticoagulation.
Methods: Through PubMed, OVID, and MEDLINE, we performed a literature review of several studies to assess whether patients with short-lived atrial fibrillation benefit from anticoagulation. Although several studies provided valuable information, we selected 6 studies that reported the duration of POAF, risks of ischemic stroke, and mortality.
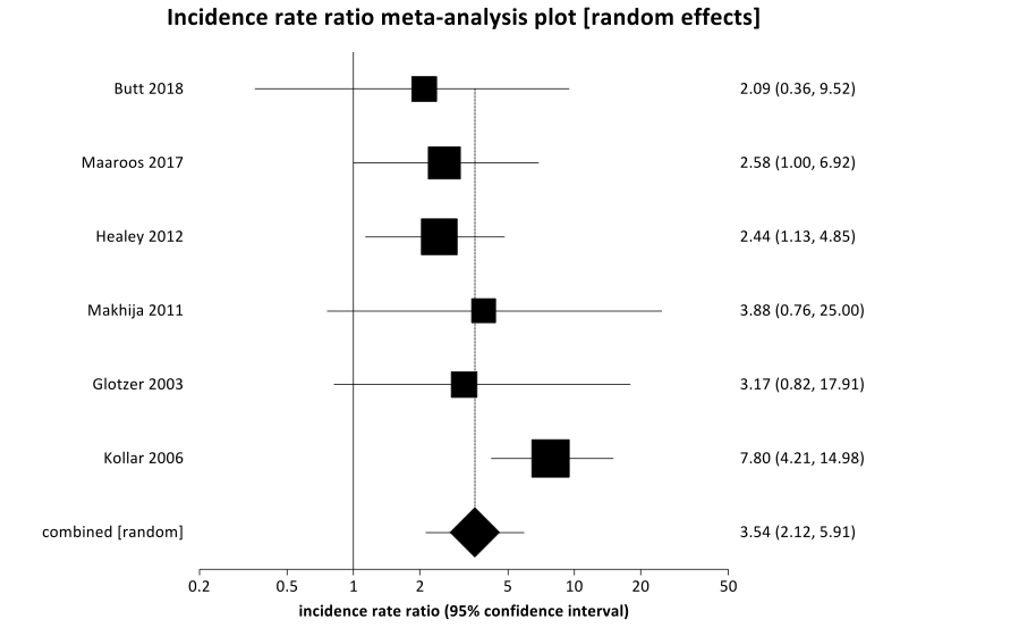
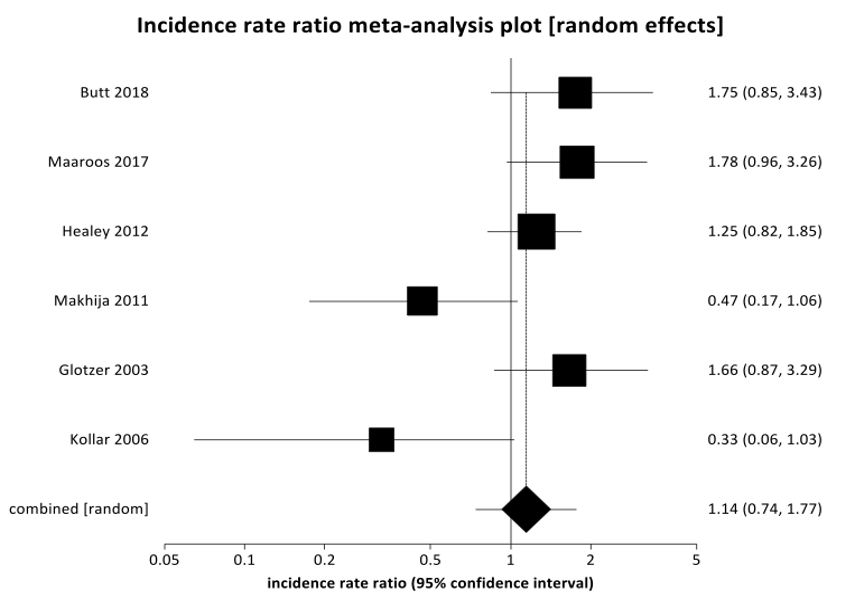
Results: We found that patients that received anticoagulation due to newly diagnosed POAF were 3.5-times less likely to experience an ischemic stroke than patients who did not receive anticoagulation with newly diagnosed POAF (IRR 3.54 (95% CI 2.12-5.91), p=0.001.). There were no statistically significant differences found in mortality outcomes between POAF patients that received anticoagulation as compared to those patients that did not receive anticoagulation (Pooled IRR = 1.1449 (95% CI = 0.738952 to 1.773857, P = 0.5447).
Conclusion: Patients with POAF over 24 hours duration were less likely to experience ischemic stroke if they were placed on anticoagulation.We hope that this meta-analysis would promote further prospective studies into the question of length of POAF and how chronic anticoagulation therapy plays a role in decreasing risks of ischemic stroke and/or mortality.
Keywords
Postoperative atrial fibrillation, atrial fibrillation, anticoagulation, perioperative atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia currently known to affect millions in the United States and billions worldwide [1]. The sequelae of AF include ischemic stroke, heart failure, and mortality, to name a few [2]. There are many risk factors that contribute to the development of AF, such as age, hypertension, obesity, ischemic heart disease, heart failure, hyperthyroidism, smoking, left atrial enlargement, and previous episodes of atrial fibrillation [3-6]. Cardiac and noncardiac surgery are also considered risk factors for the development of AF; however, this type of AF is subcategorized as “postoperative atrial fibrillation” (POAF). While POAF may be experienced by any patient that undergoes surgery, patients undergoing cardiac surgery have a greater likelihood of experiencing POAF. Generally, patients undergoing thoracic surgery are two times more likely to experience AF than patients undergoing non-thoracic surgery [7]. Additionally, a study suggested patients who develop POAF are eight times more likely to develop AF later in life [8]. Previous studies and guidelines from the AHA/ACC in 2014 provided Class IIa recommendation on initiating anticoagulation in patients with postoperative atrial fibrillation lasting over 48 hours, based on Level B evidence [9]. While there have been several studies investigating the occurrence of postoperative atrial fibrillation and its relationship to ischemic stroke and mortality, there have been limited studies discussing the duration of POAF that may be associated with increased risks of ischemic stroke and/or mortality. In this meta-analysis, we aimed to investigate the duration of POAF and compare primary outcomes of ischemic stroke and mortality in patients who received anticoagulation as opposed to patients who did not receive anticoagulation.
Methods
We performed a literature review of several studies through PubMed, OVID, and MEDLINE to assess whether or not patients with short-lived atrial fibrillation benefit from anticoagulation. Although several studies provided valuable information, we selected 6 retrospective studies from 2000 to 2019 that reported the duration of atrial fibrillation as at least > 24 hours, the timing of initiation of anticoagulation in relation to the origin of POAF, ischemic stroke events, major bleeding events, and mortality as part of our inclusion criteria. Search terms included “perioperative” OR “postoperative” AND “atrial fibrillation” AND “ischemic stroke” OR “cerebrovascular accident” OR “death” OR “mortality”. There were no language restrictions applied to search criteria. Approximately 26,284 patients were analyzed among all 6 studies. Major bleeding was later excluded as an outcome as only one included study reported outcomes regarding major bleeding [10]. Table 1 demonstrated study characteristics for POAF patients that received anticoagulation, while (Table 2) demonstrated study characteristics for patients that did not receive anticoagulation.
Table 1: Study Characteristics for Anticoagulation Patients.

Table 2: Study Characteristics for Non-Anticoagulation Patients.

Results
A total of 6 retrospective studies involving approximately 26,284 patients were included in the analysis of POAF and the relationship to ischemic stroke and mortality. Approximately 5,232 patients received anticoagulation within 30 days of onset of POAF, and 21,052 patients did not receive anticoagulation within 30 days of onset of POAF. Age and gender were matched in the included studies to minimize variations across populations. For this meta-analysis, the average age among all populations was 70.8 years among the anticoagulation group and approximately 72.2 years among the non-anticoagulation group.
Figure 1: Forest plot of pooled incidence rate ratio for ischemic stroke in postoperative atrial fibrillation indicates that the non-coagulation group (or control) experienced a higher incidence rate compared to the anticoagulation group (or treatment), IRR 3.54 (95% CI 2.12-5.91), p=0.001.
Figure 2: The pooled incidence rate ratio for mortality indicates that the non-coagulation group (or control) did not experience a higher incidence rate compared to the anticoagulation group (or treatment), IRR 1.14 (95% CI 0.74-1.77), p=0.5447.
Figure 1 shows a Forest plot of pooled incidence rate ratio regarding the primary outcomes of ischemic stroke. When these studies were combined, this meta-analysis demonstrated that non-anticoagulated patients that experienced POAF were almost three and a half times more likely to experience an ischemic stroke than patients with POAF that received anticoagulation [IRR 3.54 95% CI (2.12-5.91)] with p=0.001. There was no significant difference noted in mortality in patients with POAF that received anticoagulation as compared to patients that did not receive anticoagulation (p=0.5447). Figure 2 shows a Forest plot with respect to the primary outcome of mortality. There was insufficient data to characterize trends in major bleeding (only reported by Makhija); thus, this result was not reported in this meta-analysis. Supplementary Figure 1 summarizes the incidence rate ratio (IRR) of ischemic stroke in POAF in non-anticoagulated patients as compared to patients that have received anticoagulation in each included study. Bias plots for ischemic stroke and mortality may be found in (Supplementary Figures 2 & 3), respectively.
Discussion
In this meta-analysis of 6 retrospective studies, approximately 26,284 patients were analyzed. Each patient analyzed in the studies was found to have POAF for at least 24 hours duration. The exceptions were the Maaroos study and the Glotzer study, where patients with POAF were found to have POAF for at least 5 minutes and 6 minutes, respectively [11, 12]. Most of our patients in this study included post cardiac surgery patients, either post coronary artery bypass graft or post valvular surgery [11-14]. The exceptions were the Makhija and Butt studies, which studied patients involved in non-cardiac surgery [10, 15]. We found that patients that received anticoagulation due to newly diagnosed POAF were 3.5 times less likely to experience an ischemic stroke than patients who did not receive anticoagulation with newly diagnosed POAF. There were no statistically significant differences found in the mortality outcomes between POAF patients that received anticoagulation as compared to those patients that did not receive anticoagulation. This outcome has also been seen in a similar meta-analysis by Lin et al. (2019), without discreet discussion of the duration of POAF [16, 17]. The impact of the increased likelihood of ischemic stroke secondary to AF would suggest that POAF patients would benefit from chronic anticoagulation.
Recent studies suggest that patients may benefit from long term anticoagulation due to the developed recurrence of AF later in life [8, 16]. As noted above previously, Ahlsson et al. noted that patients with POAF are eight times more likely to experience a recurrence of AF in the future [8]. El-Chami et al. used implantable loop recorders for long-term cardiac rhythm monitoring, showing that 60.9% of patients with POAF developed recurrent atrial fibrillation (i.e., any atrial fibrillation episodes >6 minutes) [16]. This finding suggested that POAF after cardiac surgery is an independent predictor of late atrial fibrillation, with a 4-fold to 5-fold higher risk [16]. An editorial comment from Dr. Verma and Dr. Bhatt stated that although studies are promising, a consideration should be made that patients with POAF also may be sicker in general, as evidenced by baseline characteristics and increased risk factors [18]. Kosmidou et al. performed an analysis of patients that developed POAF post CABG (coronary artery bypass graft) and compared outcomes to patients post PCI (percutaneous coronary intervention) [19]. Patients that were found to have new onset AF were more likely to have pre-existing comorbid conditions like anemia, history of myocardial infarction or low left ventricular ejection fraction, which may have contributed to higher risks of development of AF [19]. While we are not dismissing the similarities seen in the baseline characteristics of patients in this study, it is still important to keep in mind the factors that may have contributed to the higher likelihood of development of POAF as well as the duration of POAF. Nonetheless, the study also found that POAF was linked with a higher incidence of ischemic stroke and mortality in post-CABG patients [19].
We hope that ongoing studies that are taking place currently (i.e., SEARCH-AF) will be able to provide a better idea on duration of POAF during patient in-hospital stays and may also help clarify whether POAF patients may benefit from long-term anticoagulation.
Limitations
There were several limitations in our meta-analysis. First, we have a small quantity of studies for meta-analysis, for example, in comparison to Lin et al., which included 35 studies. However, of note, our goal in this study was to also include duration of POAF and to identify impacts on outcomes of duration of POAF, and how they play a role in outcomes of ischemic stroke and mortality. Additionally, there were limited notations of bleeding risk and outcomes mentioned in individual studies; thus, unfortunately, we were unable to complete an analysis of bleeding as an outcome.
Conclusion
Postoperative atrial fibrillation patients of greater than 24 hours duration would likely benefit from chronic anticoagulation to prevent ischemic stroke. There were no statistically significant differences in mortality in POAF patients that received anticoagulation as compared to patients that did not receive anticoagulation that would suggest any mortality benefits. Further prospective studies should be performed to analyze the duration of POAF and the need for chronic anticoagulation.
Acknowledgements
At this time, we would like to thank Southern Illinois University School of Medicine for their support. We must specially thank the co-authors and the departments of Cardiology, Internal Medicine, and Statistics for the mentorship and guidance in designing and creating this manuscript.
Author Contributions
All noted authors above have provided significant contributions to the manuscript. Their work and dedication are greatly appreciated. The material presented in this manuscript has been prepared by all authors noted.
Disclosures
None.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 15, Apr 2020Accepted: Wed 15, Apr 2020
Published: Wed 15, Apr 2020
Copyright
© 2023 Nitin Tandan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.02.02
Author Info
Abdisamad M. Ibrahim Abhishek Kulkarni Albert Botchway Manjari Rani Regmi Mohamed Labedi Mohammad AlAkchar Momin Siddique Nitin Tandan Ruby Maini
Corresponding Author
Nitin TandanDepartment of Internal Medicine, Southern Illinois University School of Medicine, Springfield, Illinois, USA
Figures & Tables
Table 1: Study Characteristics for Anticoagulation Patients.

Table 2: Study Characteristics for Non-Anticoagulation Patients.

References
- Heart Disease, Centers for Disease Control and Prevention (2019) Atrial Fibrillation.
- Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983-988. [Crossref]
- Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM et al. (2015) Atrial Fibrillation Incidence and Risk Factors in Relation to Race-Ethnicity and the Population Attributable Fraction of Atrial Fibrillation Risk Factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 25: 71-76. [Crossref]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW et al. (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139: e56-e528. [Crossref]
- Benjamin E, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ et al. (1994) Independent risk factors for atrial fibrillation in a population-based cohort. JAMA 271: 840-844. [Crossref]
- Thijs V, Lemmens R, Farouque O, Donnan G, Heidbuchel H (2017) Postoperative atrial fibrillation: Target for stroke prevention? Eur Stroke J 2: 222–228. [Crossref]
- Rostagno C, La Meir M, Gelsomino S, Ghilli L, Rossi A et al. (2010) Atrial fibrillation after cardiac surgery: incidence, risk factors, and economic burden. J Cardiothorac Vasc Anesth 24: 952-958. [Crossref]
- Ahlsson A, Fengsrud E, Bodin L, Englund A (2010) Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg 37: 1353-1359. [Crossref]
- 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology. Elsevier 64.
- Makhija Z, Allen MS, Wigle DA, Shen KR, Cassivi SD et al. (2011) Routine anticoagulation is not indicated for postoperative general thoracic surgical patients with new-onset atrial fibrillation. Ann Thorac Surg 92: 421-427. [Crossref]
- Maaroos M, Pohjantähti Maaroos H, Halonen J, Vähämetsä J, Turtiainen J et al. (2017) New onset postoperative atrial fibrillation and early anticoagulation after cardiac surgery. Scand Cardiovasc J 51: 323-326. [Crossref]
- Glotzer, TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R et al. (2003) Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 107: 1614-1619. [Crossref]
- Kollar A, Lick SD, Vasquez KN, Conti VR et al. (2006) Relationship of atrial fibrillation and stroke after coronary artery bypass graft surgery: when is anticoagulation indicated? Ann Thorac Surg 82: 515-523. [Crossref]
- Melduni RM, Schaff HV, Bailey KR, Cha SS, Ammash NM et al. (2015) Implications of new-onset atrial fibrillation after cardiac surgery on long-term prognosis: a community-based study. Am Heart J 170: 659-668. [Crossref]
- Butt JH, Olesen JB, Havers Borgersen E, Gundlund A, Andersson C et al. (2018) Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J Am Coll Cardiol 72: 2027-2036. [Crossref]
- Lin MH, Kamel H, Singer DE, Wu YL, Lee M et al. (2019) Perioperative/Postoperative Atrial Fibrillation and Risk of Subsequent Stroke and/or Mortality. Stroke 50: 1364-1371. [Crossref]
- Ibrahim AM, Tandan N, Koester C, Al Akchar M, Bhandari B et al. (2019) Meta-analysis evaluating outcomes of surgical left atrial appendage occlusion during cardiac surgery. Am J Cardiol 124: 1218-1225. [Crossref]
- Verma A, Bhatt DL, Verma S (2018) Long-Term Outcomes of Post-Operative Atrial Fibrillation. J Am Coll Cardiol 71: 749-751. [Crossref]
- Kosmidou I, Chen S, Kappetein AP, Serruys PW, Gersh BJ et al. (2018) New-Onset Atrial Fibrillation After PCI or CABG for Left Main Disease: The EXCEL Trial. J Am Coll Cardiol 71: 738-748. [Crossref]

