A Case Report of Cerebral Vasospasm Following Elective Clipping of Unruptured Aneurysm: Pathogenetic Theories and Clinical Features of a Dangerous Underestimated Event
A B S T R A C T
Background and Importance: Angiographic-proven and clinically-evident cerebral vasospasm (CVS) after uneventful elective clipping of unruptured intracranial aneurysm (UIA) is a very rare and often underestimated event. To date, the knowledge of risk factors, pathophysiology, and demographic characteristics of these conditions are solely relegated to few case reports. With the aim of better characterize shared features and mechanism that could be involved in such event we also performed a review of the present literature and analyzed aneurysm’s features, surgical factors, treatments, recovery and of all reported cases of CVS after elective clipping.
Clinical Presentation: We report a case of a cerebral vasospasm following elective clipping of a middle cerebral artery (MCA) bifurcation aneurysm in a 59-year-old woman who smoked next days after treatment, despite medical advice. We found ten cases comparable to ours with angiographic-proven and clinically evident cerebral vasospasm after uneventful elective clipping.
Conclusion: Classic mechanisms of CVS following SAH have been widely studied. In all the cases we analyzed, no subarachnoid bleeding occurred, as demonstrated in pre and postoperative CT scans and intraoperatively. Various theories on the possible mechanism have been advanced. It seems reasonable that CVS following elective clipping of unruptured aneurysm is a multifactorial phenomenon. Although its pathogenesis is unclear, clinicians should keep in mind the existence of this event, that is rare, but it could be seen in the clinical practice of every neurosurgery ward. In our opinion, it’s worth to know this possible post-operative complication because, when suspected clinical signs and symptoms of delayed ischemic neurological deficit (DIND) arise after elective clipping, it’s important to make an early diagnosis of CVS owing to early treatments are critical to improve clinical outcome.
Keywords
Cerebral vasospasm, vascular neurosurgery, unruptured aneurysm, elective clipping
Introduction
CVS is defined as narrowing of one, or more, large or medium-sized intracranial artery caused by a persistent contraction of its muscular wall, with consequent endoluminal diameter reduction [1, 2]. This event occurs in two-thirds of patients affected by subarachnoid hemorrhage (SAH) and it usually develops around the 4th day and gradually recedes within the 21st day after SAH. Besides subarachnoid hemorrhage, CVS has been described after traumatic events, skull base surgery, meningitis, eclampsia and even in the presence of unruptured aneurysm [3-7]. In addition, CVS has been reported after elective clipping of unruptured aneurysm. The latter is a rare and often underestimated event that can develop after an apparently uncomplicated surgery. To date, the knowledge of risk factors, pathophysiology, and demographic characteristics of these conditions are solely relegated to few case reports. In the present paper, we report a case of a cerebral vasospasm following elective clipping of a MCA bifurcation aneurysm in a 59-year-old woman who smoked the days following treatment, despite medical advice. With the aim of better characterize shared features and mechanism that could be involved in such event we also performed a review of the present literature and analyzed aneurysm’s features, surgical factors, treatments, recovery and of all reported cases of CVS after elective clipping.
Table 1: All cases reported. Abbreviations: ACoA: Anterior communicating artery; CT: computed tomography; EDH: epidural haematoma; ICA: internal carotid artery; ICH: intraparenchimal haematoma; MCA: middle cerebral artery; MRI: magnetic resonance imaging; NA: Not Available
|
Case |
Author, year |
Sex |
Age (yrs) |
Comorbidities |
Aneurysm location |
Aneurysm size (mm) |
Multiple aneurysm |
Previous SAH |
CVS onset (postoperative days) |
CVS location |
Temporary clipping (min) |
Number of clips |
Other relevant surgical details |
Relevant Imaging (CT/MRI) |
Symptoms
|
Treatment |
Outcome |
|
1 |
Bloomfield, 1985 |
F |
54 |
NA |
right MCA bifurcation |
7 |
|
|
9th |
|
NA |
2 |
aneurysm puncture after clipping, transient MCA focal spasm |
none |
left hemiparesis |
hydratation dexamethasone 4 mg/hrs for 5 days |
Partial recovery |
|
2 |
Gutierrez, 2001 |
F |
55 |
NA |
left ICA (ophtalmic segment) |
3\5 |
no |
NA |
1st (16 hrs) |
M1,M2, A1,A2 |
NA |
NA |
NA |
none |
severe right hemiparesis, aphasia, coma |
papaverine instillation |
Partial recovery (slight right hemiparesis) |
|
3 |
Kitazawa, 2004 I |
F |
21 |
NA |
left ICA (paraclinoid) |
4 |
no |
no |
12th |
left M2 |
use (unknown, several times) |
1 straight (Sugita no.2) |
bleeding fron cavernous sinus |
none |
aphasia, Gerstmann |
chemical angioplasty (papaverine hydrochloride 80 mg), triple H |
Complete recovery |
|
4 |
Kitazawa, 2004 II |
F |
63 |
NA |
left ICA (paraclinoid) |
5 |
no |
no |
9th |
NA |
use (unknown) |
2 |
none |
mild EDH (unknown if it requires surgery) |
aphasia, right hemiparesis |
chemical angioplasty (unknown), triple H |
Complete recovery |
|
5 |
Paolini, 2005 |
F |
47 |
smoking (2/3 packs a day) |
right MCA bifurcation |
8 |
|
|
28th |
distal M1 |
use (unknown) |
2 straights |
none |
postop CT scan:right f-t ICH with brain swelling; on 28th day CT:subacute infarction in tha MCA periventricular areas |
left faciobrachiocrural hemiparesis |
hydratation, antiplatelet |
Complete recovery |
|
6 |
Yang, 2011 |
F |
41 |
NA |
left ICA bifurcation |
5 |
|
|
28th |
left distal ICA,A1 and M1 |
use (3 (su art hr??errore?) |
2 |
none |
none |
aphasia, right facial numbness |
hydratation, antiplatelet, chemical angioplasty (unknown drug) |
partial recovery (minimal aphasia) |
|
7 |
Yang, 2012 |
F |
61 |
NA |
left MCA bifurcation |
6 |
|
|
10th |
NA |
use (?) |
3 |
none |
none |
aphasia, mental change |
hydratation, antiplatelet, chemical anicardipina 8 mg) |
partial recovery (minimal aphasia) |
|
8 |
Hashimoto, 2015 |
F |
62 |
NA |
left ICA-PCA |
5 |
|
|
11th (radiological 8th) |
diffuse:bilateral A1, left M!, right M3-M4, bilateral PCA |
no |
NA |
residual neck wrapped by temporal muscle graft |
none the postopCT; a Ct scan performed at 8th postop day showed M1 vasospasm+left hemispheric edema, 11th day MRI: left MCA ictus |
disorientation, aphasia, right hemiplegia |
hypervolemic+antiplatelet therapy |
partial recovery (acalculia, paraphasia) |
|
9 |
Tsyben, 2016 |
F |
53 |
NA |
left M1 |
5 |
|
|
2nd (30 hrs) |
left MCA |
no |
1 |
reposition of clip 3 times |
none, until MRI on the 6th postop day:left MCA stroke |
dysphasia, fluctuant right hemiparesis (> upper limb) |
chemical angioplasty (verapamil) |
Partial recovery (minimal dysphasia, right upperl limb paresis) |
|
10 |
Tsyben, 2016 |
M |
70 |
DM type II, essential hypertension, smoking |
ACoA, two small left MCA |
NA |
yes |
|
2nd |
left M2 and M3 |
yes (both A1 for 4min) |
unknown |
old xantochromic arachnoid staining near one small MCA an., resection of gyrus rectus |
none |
decrease of consciousness, aphasia, right hemiparesis |
chemical angioplasty (verapamil 20 mg), 3 days of hypertnesion and hypervolemia, 21day of nimodipine |
Complete recovery |
|
11 |
Present Case |
F |
59 |
smoking (2/3 packs a day), hypertension |
left MCA bifurcation |
5 |
yes: 3mm left ICA |
no |
6th |
left M1 and MCA bifurcation |
yes (M1 for 6min) |
1 curved Sugita clip |
none |
left frontobasal and temporal ipodensity on postop CT scan |
aphasia, right hemiparesis |
chemical angioplasty (nimodipine) |
Partial recovery |
Material and Methods
A search of MEDLINE from 1985 to present has been performed. Search terms were: “cerebral vasospasm” and “unruptured aneurysm” and “after elective aneurysm clipping”. Inclusion criteria were: 1) No evidence of a ruptured aneurysm or SAH in pre-operative CT scan and intraoperatively, 2) clinically evident vasospasm, 3) angiographically proven vasospasm. We found ten cases comparable to ours with angiographic-proven and clinically evident cerebral vasospasm after uneventful elective clipping [2, 8-14]. All cases, together with our, are summarized in (Table 1).
Case Description
A 59-year-old. female was admitted for elective clipping of a 5 mm left MCA bifurcation aneurysm (Figure 1 & Figure 2). She had a history of hypertension and she was a heavy smoker with a consumption of 30 cigarettes per day for since she was 30-year-old. The aneurysm was incidentally discovered during the investigation for an occasional headache and dizziness. Exams revealed also another smaller aneurysm located at the left internal carotid artery (ICA) (about 3 mm of maximum diameter) regarding which a conservative approach was chosen. No clinical or radiological signs of previous SAH were detected during preoperative workup. The patient underwent a pterional craniotomy for the exposition of the left MCA aneurysm, which was performed through a proximal-to-distal dissection. The aneurysm was clipped with a 7 mm Sugita curved clip, a temporary six-minutes-long M1 clipping was performed. There were no vessels sacrifice, nor intraoperative evidence of bleeding. The patency of the parent vessels and the complete aneurysm exclusion were verified with an intraoperative Doppler exam. Local papaverine was used as per local protocol. The postoperative neurological exam was negative, and the patient quickly resumed the ability to walk yet on the 2nd postoperative day. Since that moment, against medical advice, the patients smoked many cigarettes (about 4 per day). A first postoperative computer tomography (CT) scan scheduled for the 6th postoperative day was negative for SAH and showed only a small hypodensity in the left front - basal and temporal area of the brain, not associated with any clinical events. On the same day, she developed motor aphasia with right side hemiparesis and underwent a new CT scan, that resulted unchanged compared to the previous one.
Figure 1: 3D angiogram showing a left MCA bifurcation aneurysm and a left ICA aneurysm.
Figure 2: Angiogram showing a left MCA bifurcation aneurysm (white arrow).
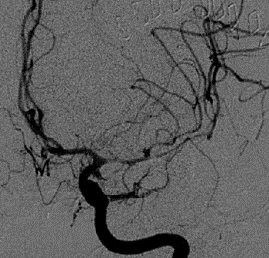
Figure 3: Angiogram showing a narrowing on M1 tract of left MCA.
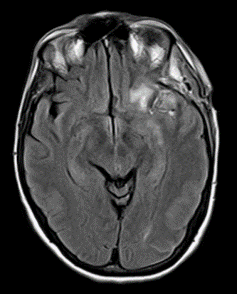
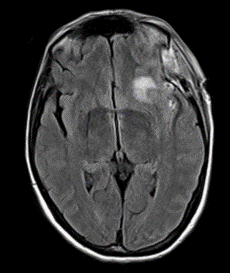
Considering a possible diagnosis of vasospasm, the patient underwent a cerebral angiography that confirmed an initial vasospasm of the left M1 and MCA bifurcation (Figure 3). An urgent chemical angioplasty with nimodipine was therefore performed (Figure 4). A subsequent cerebral magnetic resonance imaging (MRI) carried out a few hours later showed initials signs of parenchymal suffering on ischemic basis (Figure 5 & Figure 6). The next CT exam, performed on the 8th postoperative day, showed a front insular ischemic area, associated with a left MCA’s diameter reduction. The vasospasm was then followed with daily transcranial Doppler (TCD) exams (initial values on left M1 250mm/s; MCA bifurcation 270 mm/s) showing a progressive normalization of the values in about two weeks. The patient was discharged on the 25th postoperative day with improved clinical signs. At the time of discharge, she was able to walk independently; the speech had phonemic and nominal paraphasia and occasional speech arrest. On long-term follow up (one year) the patient further improved, and she was able to return to work.
Figure 4: Light reduction of cerebral vasospasm on M1 tract of left MCA after chemical angioplasty with nimodipine.
Figure 5: MRI FLAIR sequence showing an ischemic area in the left MCA territory.
Figure 6: Other MRI FLAIR sequence showing a front insular ischemic area.
Results
Among eleven patients ten patients were female, only one was a male; this is probably due to the greater number of women that suffers from UIA compared to men. The mean patient’s age was 53, 2 years ranged (21-70 years). Three cases were reported to be smokers. Our case was the sole in which early smoking during hospitalization is reported. One patient suffered type II diabetes and hypertension also. The mean aneurysm size was 5, 4 mm and all were in the anterior circulation with left prevalence ranged (4-8 mm, 5 at left ICA, 2 at right MCA bifurcation, 3 at left MCA bifurcation/M1 and 1 at anterior communicating artery (ACoA)). Only two patients had associated aneurysms (3 mm left ICA aneurysm in our case and two small left MCA aneurysms in case number 10).
No patients suffered previous SAH or exhibited intraoperative or radiological sign of bleeding (authors of the case no. 10 reported the presence of old xanthochromic arachnoid staining near one small MCA aneurysm, but no evidence of any recent SAH).Regarding the surgical technique, temporary clipping was performed in 7 patients and multiple clips (mean value 2, 2 clips) were used in 5 cases. Peculiar intraoperative events included transient MCA focal spasm after post-clipping aneurysm puncture in one case, cavernous sinus bleeding in another case, wrapping with a temporal muscle graft of the residual neck of the aneurysm was performed in the case reported by Hashimoto et al. and the definitive clip was repositioned 3 times in the ninth case and in 1 time in our case [8, 12, 14]. The onset of CVS was reported to occur between 1 and 28 days following surgical treatment with the mean time to onset of 10, 6 days (median 9 days).
Vasospasm was diffuse in only two cases, meanwhile in the others it was localized near the site of surgery in the proximal segments of ICA, MCA and anterior cerebral artery (ACA) (2 in distal ICA, 6 in M1, 3 in M2, 3 in A1, 1 in A2 and 1 in M3). The symptoms included aphasia in 9 patients, right hemiparesis in 5 patients, left hemiparesis in 2 patients, right hemiplegia in 1 patient, Gerstmann’s syndrome in 1 patient, disorientation in 1 patient, decrease of consciousness in 1 patient. CVS treatment included chemical angioplasty (2 patients with verapamil, 2 patients with papaverine, 1 patient with nimodipine, 1 patient with nicardipine and 2 patients with unknown drugs), triple H therapy, hydration and antiplatelet therapy. After treatment, 4 patients had a complete recovery and 7 patients had a partial recovery.
Discussion
Classic mechanisms of CVS following SAH have been widely studied. The hemoglobin released from subarachnoid blood clots triggers intracellular entry and release of calcium with subsequent actin and myosin cross-linkage and vasal smooth muscle contraction [15]. The contractile proteins protein kinase C, Rho kinase, and protein tyrosine kinase and their corresponding signal transduction pathways have been implicated in vasospasm models when their activation shifts the contractile mechanism toward increased shortening in the absence of high intracellular calcium levels. The presence of reactive oxygen species (ROS) and free hemoglobin induce the reduction of nitric oxide (NO) production by the endothelial cells, which has been demonstrated to have a direct effect on arterial smooth muscle contraction and in the regulation of regional cerebral flow [11, 13]. Endothelin-1 (ET-1) has the greatest role in vasoconstriction; ET-1 levels have been found to be elevated in the cerebrospinal fluid (CSF) of patients in whom vasospasm and brain ischemia develops.
However, these mechanisms are implicated in presence of subarachnoid blood around arterial vessels. In all the cases we analyzed, no subarachnoid bleeding occurred, as demonstrated in pre and postoperative CT scans and intraoperatively. Various theories on the possible mechanism have been advanced summarized in (Table 2).
Table 2: Proposed Theories of CVS.
|
|
Proposed Theories of CVS |
|
1 |
Intraoperative bleeding around vassels |
|
2 |
Spasmogenic blood breakdown products from aneurysm sac |
|
3 |
Allergic reaction to metal’s clip |
|
4 |
“hypothalamic” theory |
|
5 |
Trigemino-cerebrovascular reflex (TCVS) |
|
6 |
Mechanical stress (temporary clipping, multiple clips, etc.) |
During elective surgery, certainly cerebral arteries are exposed to a small amount of blood and although some cases of CVS after resection of skull base tumors or pituitary adenomas with a small amount of postoperative SAH surrounding blood vessels are reported, it seems to be unlikely that the amount of intraoperative extravasated blood would be alone responsible for CVS, as supported by the wide temporal range of vasospasm onset reported (1-28 days) [8, 13].
Other factors may be implicated, and many hypotheses have been proposed. DeLong, based on the clinical observation that resecting rather than simply closing the aneurysm sac seems to be accompanied by a lower incidence of postoperative vasospasm, hypothesized that spasmogenic blood breakdown products might diffuse into the arterial wall not only from the subarachnoid cisterns but also from the inside of the aneurysm once this has been secured [16]. This presumes a very long process, but the early time of onset and the extremely rare occurrence of the CVS after elective clipping would seem to make this mechanism unlikely, except for the cases in which was observed a delayed CVS (Table 1).
Hashimoto et al, in light with the existence of transient vasculitis caused by allergic reaction to a metal, like nickel and titanium, tested their patient for allergic reactions to 3 different clips but no cases resulted positive [14]. We used a 7 mm Sugita curved clip. We did not test our patient for allergic reaction to metal, but she has negative allergy history. Also, in the other cases, there was not reported any history of allergy, so this event seems to be much rarer than CVS after elective clipping itself.
Some authors advocated the “hypothalamic” theory. According to this theory, mechanical or vascular impairment of the hypothalamus could promote the release of vasospastic mediators [2, 8-10]. If this hypothesis could explain CVS in aneurysms arising from structures near the midline (such as ACoA or paraclinoid tract of ICA), on one other hand, it seems less conceivable for CVS after clipping of more peripheral aneurysm (as in our case) on the other.
It is demonstrated that meninges and cranial vessels receive a trigeminal innervation, especially through the ophthalmic branch [17]. This trigeminocerebrovascular system (TCVS) is part of a complex nerve network surrounding the arteries of the circle of Willis. It seems to be involved in maintaining a normal vessel diameter in response to arterial vasoconstriction by a constant release of vasodilatory peptides such as substance P and calcitonin gene-related peptide (CGRP) [9]. The concentration of CGRP in the external jugular venous blood and CSF strictly correlate with the grade of vasospasm revealed by transcranial Doppler [13].
Hypothetically, stimulation of the TCVS nerve by chemical factors, i.e. hemoglobin or prostaglandins, o mechanical stretching of the arterial wall could produce a failure of the “trigemino-cerebrovascular reflex” with onset of CVS [9, 13].
An interesting aspect about CVS pathogenesis is endothelial damage produced by mechanical factors. Aggressive manipulation of the vessel wall during surgery may cause a distention or a damage in the endothelium, that could induce an imbalance between vasoactive agents, with the preponderance of the vasoactive ones, like in the post-SAH CVS [13]. Therefore, it could be argued that local pressure of the clips on aneurysm wall or occlusion of a vessel by a temporary clip, may produce endothelial damage. The vascular stress may be severe especially in the case of use of multiple clips or if there are multiple attempts before the definitive clipping or if the temporary clipping last longer. Kitazawa et al. found a statistically significant correlation between CVS after elective clipping and the number of clips used and temporary occlusion of the ICA [8].
On the other hand, a recent paper of Malinova et al found that temporary clipping did not contribute to a higher rate of TCD-vasospasm, DIND, or delayed cerebral ischemia (DCI) in comparison with rates in patients without temporary clipping; it’s worth to mention that this study included only patient underwent a clipping of ruptured cerebral aneurysm and so a different context compared to our case [18]. The use of a temporary clip or multiple clips is a common practice in aneurysm surgery. In our case, we used a temporary clip for 6 minutes and the definitive clip was replaced 1 time. In our analysis, only in 7 cases a temporary clip was used, and multiple clips were used in 5 cases. In our opinion, it would be interesting to understand if the time of use of a temporary clip or the numbers of attempts to place the definitive clip could be statistically correlated with CVS. Moreover, in our case the exposition of the UIA was done thanks to a proximal-to-distal dissection, and this could have increased the surgical manipulation of the vessel and, indeed the endothelial damage due to mechanical factors.
Secondly, areas of turbulence within the aneurysm could produce mechanical stress on the vascular wall with consequent endothelial damage and NO production impairment [19]. Also, ROS could be produced in this area of turbulent flow because of the presence of relatively hypoxic blood [20]. At the same time, several experimental studies demonstrated that, apart from causing vascular endothelial dysfunction, cigarette smoke causes physical damage to the vascular endothelium by contraction or death of endothelial cells [21]. Chronic exposure to cigarette smoking might damage the blood-brain barrier (BBB) and inflammatory effects produced by exposure to cigarette smoke may favor perivascular inflammation. In two previous case was reported smoking habit, but we have no information about the temporal correlation between smoking and onset of symptoms. In our case, the patient smoked few days after she underwent surgical clipping. This early exposure to cigarette smoke may have contributed and amplified the endothelial alteration produced by the set of events mentioned above, even if probably the acute effect of smoke on the endothelial alteration of the arteries is much less important that the chronic one (promotion of atherosclerosis and alteration of vessel function).
The paper presents several limitations. Firstly, the incidence of CVS after clipping of UIA is unpredictable due to the lack of data regarding the amount of surgical clipping performed every year in Europe or US. It could be helpful create a nationwide register of UIA clipping procedures. Secondly, the limited number of the reported cases prevent to make any statistical analysis. As well as what concern CVS following SAH, an early diagnosis and treatment could be reasonably suggested for the management of CVS after elective clipping, despite the limited numbers and the various treatment performed in reported cases.
Conclusion
It seems reasonable that CVS following elective clipping of unruptured aneurysm is a multifactorial phenomenon involving some mechanical and biochemical factors cause of vascular injury, production of vasoactive agents and failure of arterial wall response. Although the pathogenesis is unclear, clinicians should keep in mind the existence of this event, that is rare, but it could be seen in the clinical practice of every neurosurgery ward. Indeed, in our opinion, it’s worth to know this possible post-operative complication because, when suspected clinical signs and symptoms of DIND arising after elective clipping, early diagnosis and treatments seem critical to improve clinical outcome.
Ethics Approval and Consent to Participate
Ethics approval and consents were not needed as this study was retrospective and all data were de-identified.
Consent for Publication
Consent for publication was obtained from all individual participants included in the study.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare that they have no competing interests.
Funding
None.
Author Contribution
Marco Ceraudo and Monica Truffelli conceived of the presented idea. Pasquale Anania and Alessandro Prior developed the theory and performed the computations. Monica Truffelli and Marco Ceraudo wrote the manuscript with support from Pietro Fiaschi. Alessandro D’Andrea and Gianluigi Zona supervised the project. All authors discussed the results and contributed to the final manuscript.
Conflicts of Interest
None.
Abbreviations :
ACA: Anterior cerebral artery.
ACoA: Anterior communicating artery.
BBB: Blood brain barrier.
CGRP: Calcitonin gene related peptide.
CSF: Cerebrospinal fluid.
CT: Computed tomography.
CVS: Cerebral vasospasm.
IND: Delayed ischemic neurological deficit.
ET-1: Endothelin-1.
ICA: Internal carotid artery.
MCA: Middle cerebral artery.
MRI: Magnetic resonance imaging.
NO: Nitric oxide.
ROS: Reactive oxygen species.
SAH: Subarachnoid haemorrhage.
TCVS: Trigeminocerebrovascular system.
UIA: Unruptured intracranial aneurysm.
Article Info
Article Type
Case ReportPublication history
Received: Wed 08, Jan 2020Accepted: Wed 22, Jan 2020
Published: Tue 28, Jan 2020
Copyright
© 2023 Pietro Fiaschi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.01.11
Author Info
Anania Pasquale Ceraudo Marco D’Andrea Alessandro Pietro Fiaschi Prior Alessandro Truffelli Monica Zona Gianluigi
Corresponding Author
Pietro FiaschiDivision of Neurosurgery, IRCCS Ospedale Policlinico San Martino, Genova
Figures & Tables
Table 1: All cases reported. Abbreviations: ACoA: Anterior communicating artery; CT: computed tomography; EDH: epidural haematoma; ICA: internal carotid artery; ICH: intraparenchimal haematoma; MCA: middle cerebral artery; MRI: magnetic resonance imaging; NA: Not Available
|
Case |
Author, year |
Sex |
Age (yrs) |
Comorbidities |
Aneurysm location |
Aneurysm size (mm) |
Multiple aneurysm |
Previous SAH |
CVS onset (postoperative days) |
CVS location |
Temporary clipping (min) |
Number of clips |
Other relevant surgical details |
Relevant Imaging (CT/MRI) |
Symptoms
|
Treatment |
Outcome |
|
1 |
Bloomfield, 1985 |
F |
54 |
NA |
right MCA bifurcation |
7 |
|
|
9th |
|
NA |
2 |
aneurysm puncture after clipping, transient MCA focal spasm |
none |
left hemiparesis |
hydratation dexamethasone 4 mg/hrs for 5 days |
Partial recovery |
|
2 |
Gutierrez, 2001 |
F |
55 |
NA |
left ICA (ophtalmic segment) |
3\5 |
no |
NA |
1st (16 hrs) |
M1,M2, A1,A2 |
NA |
NA |
NA |
none |
severe right hemiparesis, aphasia, coma |
papaverine instillation |
Partial recovery (slight right hemiparesis) |
|
3 |
Kitazawa, 2004 I |
F |
21 |
NA |
left ICA (paraclinoid) |
4 |
no |
no |
12th |
left M2 |
use (unknown, several times) |
1 straight (Sugita no.2) |
bleeding fron cavernous sinus |
none |
aphasia, Gerstmann |
chemical angioplasty (papaverine hydrochloride 80 mg), triple H |
Complete recovery |
|
4 |
Kitazawa, 2004 II |
F |
63 |
NA |
left ICA (paraclinoid) |
5 |
no |
no |
9th |
NA |
use (unknown) |
2 |
none |
mild EDH (unknown if it requires surgery) |
aphasia, right hemiparesis |
chemical angioplasty (unknown), triple H |
Complete recovery |
|
5 |
Paolini, 2005 |
F |
47 |
smoking (2/3 packs a day) |
right MCA bifurcation |
8 |
|
|
28th |
distal M1 |
use (unknown) |
2 straights |
none |
postop CT scan:right f-t ICH with brain swelling; on 28th day CT:subacute infarction in tha MCA periventricular areas |
left faciobrachiocrural hemiparesis |
hydratation, antiplatelet |
Complete recovery |
|
6 |
Yang, 2011 |
F |
41 |
NA |
left ICA bifurcation |
5 |
|
|
28th |
left distal ICA,A1 and M1 |
use (3 (su art hr??errore?) |
2 |
none |
none |
aphasia, right facial numbness |
hydratation, antiplatelet, chemical angioplasty (unknown drug) |
partial recovery (minimal aphasia) |
|
7 |
Yang, 2012 |
F |
61 |
NA |
left MCA bifurcation |
6 |
|
|
10th |
NA |
use (?) |
3 |
none |
none |
aphasia, mental change |
hydratation, antiplatelet, chemical anicardipina 8 mg) |
partial recovery (minimal aphasia) |
|
8 |
Hashimoto, 2015 |
F |
62 |
NA |
left ICA-PCA |
5 |
|
|
11th (radiological 8th) |
diffuse:bilateral A1, left M!, right M3-M4, bilateral PCA |
no |
NA |
residual neck wrapped by temporal muscle graft |
none the postopCT; a Ct scan performed at 8th postop day showed M1 vasospasm+left hemispheric edema, 11th day MRI: left MCA ictus |
disorientation, aphasia, right hemiplegia |
hypervolemic+antiplatelet therapy |
partial recovery (acalculia, paraphasia) |
|
9 |
Tsyben, 2016 |
F |
53 |
NA |
left M1 |
5 |
|
|
2nd (30 hrs) |
left MCA |
no |
1 |
reposition of clip 3 times |
none, until MRI on the 6th postop day:left MCA stroke |
dysphasia, fluctuant right hemiparesis (> upper limb) |
chemical angioplasty (verapamil) |
Partial recovery (minimal dysphasia, right upperl limb paresis) |
|
10 |
Tsyben, 2016 |
M |
70 |
DM type II, essential hypertension, smoking |
ACoA, two small left MCA |
NA |
yes |
|
2nd |
left M2 and M3 |
yes (both A1 for 4min) |
unknown |
old xantochromic arachnoid staining near one small MCA an., resection of gyrus rectus |
none |
decrease of consciousness, aphasia, right hemiparesis |
chemical angioplasty (verapamil 20 mg), 3 days of hypertnesion and hypervolemia, 21day of nimodipine |
Complete recovery |
|
11 |
Present Case |
F |
59 |
smoking (2/3 packs a day), hypertension |
left MCA bifurcation |
5 |
yes: 3mm left ICA |
no |
6th |
left M1 and MCA bifurcation |
yes (M1 for 6min) |
1 curved Sugita clip |
none |
left frontobasal and temporal ipodensity on postop CT scan |
aphasia, right hemiparesis |
chemical angioplasty (nimodipine) |
Partial recovery |
Table 2: Proposed Theories of CVS.
|
|
Proposed Theories of CVS |
|
1 |
Intraoperative bleeding around vassels |
|
2 |
Spasmogenic blood breakdown products from aneurysm sac |
|
3 |
Allergic reaction to metal’s clip |
|
4 |
“hypothalamic” theory |
|
5 |
Trigemino-cerebrovascular reflex (TCVS) |
|
6 |
Mechanical stress (temporary clipping, multiple clips, etc.) |



References
- Sobey CG, Faraci FM (1998) Subarachnoid haemorrhage: what happens to the cerebral arteries? Clin Exp Pharmacol Physiol 25: 867-876. [Crossref]
- O Gutiérrez, J G M P Caldas, J P Rabello (2001) Unruptured Aneurysm: Vasospasm after Surgery and Endovascular Treatment. A Case Report. Interv Neuroradiol 7: 37-39. [Crossref]
- Zurynski YA, Dorsch NW (1998) A review of cerebral vasospasm. Part IV. Post-traumatic vasospasm. J Clin Neurosci 5: 146-154. [Crossref]
- Abuzayed B, Al-Abadi H, Al-Otti S, Baniyaseen K, Al-Sharki Y (2014) Neuronavigation-guided endoscopic endonasal resection of extensive skull base mucormycosis complicated with cerebral vasospasm. J Craniofac Surg 25: 1319-1323. [Crossref]
- Lyons EL, Leeds NE (1967) The angiographic demonstration of arterial vascular disease in purulent meningitis. Report of a case. Radiology 88: 935-938. [Crossref]
- Tsukimori K, Ochi H, Yumoto Y, Iwasaki S, Hojo S et al.(2008) Reversible posterior encephalopathy syndrome followed by MR angiography-documented cerebral vasospasm in preeclampsia-eclampsia: report of 2 cases. Cerebrovasc Dis 25: 377-380. [Crossref]
- Friedman P, Gass HH, Magidson M (1983) Vasospasm with an unruptured and unoperated aneurysm. Surg Neurol 19: 21-25. [Crossref]
- Kitazawa K, Hongo K, Tanaka Y, Oikawa S, Kyoshima K et al (2005) Postoperative vasospasm of unruptured paraclinoid carotid aneurysms: analysis of 30 cases. J Clin Neurosci 12: 150-155. [Crossref]
- Paolini S, Kanaan Y, Wagenbach A, Fraser K, Lanzino G (2005) Cerebral vasospasm in patients with unruptured intracranial aneurysms. Acta Neurochir (Wien) 147: 1181-1188. [Crossref]
- Yang K, Ahn JS, Park JC, Kwon DH, Kwun BD (2015) Clinical and Angiographical Delayed Cerebral Vasospasms After Uncomplicated Surgical Clipping of Unruptured Intracranial Aneurysms: Illustrated Review and Two Case Reports. Turk Neurosurg 25: 662-665. [Crossref]
- Mayberg MR, Okada T, Bark DH (1990) The role of hemoglobin in arterial narrowing after subarachnoid hemorrhage. J Neurosurg 72: 634-640. [Crossref]
- Bloomfield SM, Sonntag VK (1985) Delayed cerebral vasospasm after uncomplicated operation on an unruptured aneurysm: case report. Neurosurgery 17: 792-796. [Crossref]
- Tsyben A, Paldor I, Laidlaw J (2016) Cerebral vasospasm and delayed ischaemic deficit following elective aneurysm clipping. J Clin Neurosci 34: 33-38. [Crossref]
- Hashimoto H, Kameda M, Yasuhara T, Date I (2015) A Case of Unexpected Symptomatic Vasospasm after Clipping Surgery for an Unruptured Intracranial Aneurysm. J Stroke Cerebrovasc Dis 25: 25-27. [Crossref]
- Kokkoris S, Andrews P, Webb DJ (2012) Role of calcitonin gene-related peptide in cerebral vasospasm, and as a therapeutic approach to subarachnoid hemorrhage. Front Endocrinol (Lausanne) 3: 135. [Crossref]
- DeLong WB (1980) Severe vasospasm with an unruptured aneurysm. Neurosurgery 6: 729. [Crossref]
- Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J, (1985) Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett; 62: 131-136. [Crossref]
- Malinova V., Shatlo B, Voit M, Suntheim P, Rohde V et al. (2017) The impact of temporary clipping during aneurysm surgery on the incidence of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Neurosurg 129: 84-90 [Crossref]
- Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M et al. (1995) Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res 76: 536-543. [Crossref]
- Harrison DG, Widder J, Grumbach I, Chen W, Weber M et al. (2006) Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med 259: 351-363. [Crossref]
- Messner B, Bernhard D (2014) Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol; 34: 509-515. [Crossref]
