A Case of Simultaneous Liver Transplantation and Mitral Valve Replacement in a HCV Cirrhotic Patient with Severe Mitral Valve Stenosis
A B S T R A C T
We report a case of simultaneous liver transplantation and mitral valve replacement in a 53 years old woman suffering for a severe HCV - related liver cirrhosis (CHILD C12, MELD 26), severe mitral stenosis and chronic atrial fibrillation. Cardiac surgery was performed on cardiopulmonary bypass with full heparinization and cardiac arrest and preceded liver transplantation. The patient was weaned on postoperative day 2 from mechanical ventilation and on postoperative day 5 from hemodynamic support and renal replacement therapy. She was transferred to the ward on POD 12. On POD 20 she was readmitted to ICU for acute kidney failure, worsening graft function, sepsis and the suspicion of acute rejection. On POD 56 after a long intensive treatment, the patient eventually died from an intracranial hemorrhage. This complex combined surgery carries a high burden of morbidity and mortality but showed a good result in the short term.
Keywords
Liver transplantation, mitral valve replacement, cerebral hemorrhage
Introduction
Liver transplantation (OLT) is the worldwide-accepted gold standard therapy for the end-stage liver disease of any aetiology: results have markedly improved over the years, one-year survival rate being close to or over 90% [1, 2]. Regardless of the aetiology of the underlying pathology, patients eligible for OLT should have a good cardiac reserve, due to the relevant hemodynamic stress imposed by the transplant surgery. Acute blood losses, massive transfusion and large use of inotropes and pressors are often recorded during (and after) OLT, making high the burden for a sick heart and adversely affecting preexistent cardiac dysfunctions [3]. Mitral valve stenosis (MVS), nowadays relatively rare in the Western world, remains a severe condition, particularly for OLT candidates [4, 5]. The hemodynamic changes caused by MVS are driven by a pressure overload starting from the left atrium, transmitted through the pulmonary circulation to the right heart, and ultimately affecting the venous system and hepatic and splanchnic circulation [6]. Among the available treatment options, mitral valve replacement (MVR) and mitral commissurotomy are the most frequently performed procedures, together with transcatheter mitral valve replacement (TMVR) [7]. However, cardiac surgery in patients with severely impaired liver function is associated with a high rate of major complications [8]. Hereby we report the case of a simultaneous intervention of MVR and OLT in a patient with severe mitral stenosis and end-stage liver disease due to HCV cirrhosis.
Case Description
A 53 years old female patient with severe HCV related liver cirrhosis diagnosed ten years before (Child C12, MELD 26, NaMELD 30) and severe mitral stenosis, was considered for a simultaneous procedure of MVR and OLT. Past medical history included: chronic atrial fibrillation, thrombocytopenia (in the setting of end-stage liver disease), brain haemorrhage twenty years before (with complete functional recovery), a long history of mitral valve disease (included a mitral valve commissurotomy twenty years before) and an episode of pulmonary embolism. Six months before surgery she became symptomatic for dyspnea for mild efforts (NYHA class III). Preoperative echocardiography showed preserved left ventricular ejection fraction, severe mitral valve stenosis (area of 1 cm2) and moderate tricuspid valve regurgitation, with pulmonary hypertension (estimated pulmonary systolic pressure =50 mmHg), dilated, mildly hypokinetic right ventricle. The surgical indication was to replace the mitral valve. Her chronic medications included furosemide, potassium canrenoate, digoxin, atenolol, and rifamixin. Preoperative functional laboratory tests were INR 2.3, total bilirubin 8.66 mg/dl, creatinine 1.26 mg/dl, urea 59 mg/dl, sodium 129 mmol/l, lactate 3 mmol/l, platelets 72000/µl.
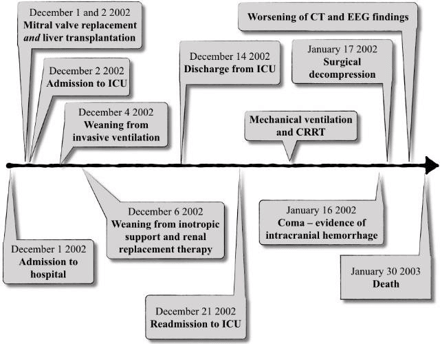
The timeline of this case is shown in Figure 1. Cardiac surgery started first and lasted 4 hours. After sternotomy access and systemic heparinization, aortic root and bicaval cannulation were performed. Cardiopulmonary bypass (CPB) was started under full heparinization. The cardioplegic arrest was induced with antegrade cold blood cardioplegia. Due to a surgical strategic choice, the mitral valve was replaced with a mechanic prosthesis, and tricuspid valve annuloplasty was also performed. Inotropic support was necessary to wean the patient from CPB and included 5 µg/kg/min of dopamine and 0,05 µg/kg/min of epinephrine. The CPB total time was 105 minutes, with 80 minutes of aortic cross-clamping. After the complete weaning from CPB support, heparin was reversed (protamine in a 1 to 1 ratio), the patient was warmed to a central body temperature of 35° and OLT was started in good hemodynamic equilibrium: Piggy-back technique without venovenous bypass and end-to-end choledochocholedochostomy were used. After reperfusion, inotropic support included epinephrine boluses (50µg + infusion up to 0,08 µg/kg/min) and dopamine up to 8 µg/kg/min.
Figure 1: The timeline of the events in the case presented.
Post-reperfusion coagulopathy was corrected (according to the thromboelastographic findings) with Protamine, Fresh Frozen Plasma (9 units) Cryoprecipitate (2 aphereses) and Platelets (1 apheresis unit). Blood losses during OLT were close to 1 liter, corrected with 3 units of packed red cells (PRC). After surgery the patient was admitted to ICU: early postoperative circulatory function support included high dose catecholamines and, because of right ventricle failure, inhalational nitric oxide. The rhythm control was achieved with epicardial pacing, amiodarone, and digoxin. Invasive mechanical ventilation was necessary until the postoperative day (POD) 2 when the patient was extubated. The renal function, poor in the immediate postoperative period, required continuous renal replacement therapy (CRRT) until POD 5. Functional assessment of the newly grafted liver included (but was not limited to) AST / ALT (AST peak 3209 u/l), INR / aPTT (highest INR value 4.79), bilirubin (peak 12.58 mg/dl). Immunosuppressive therapy consisted of methylprednisolone, anti-thymocyte globulins (first five days, 0.75 mg/kg/day) and cyclosporine (after recovery of renal function).
The postoperative anticoagulation was based on a dicumarolic agent, after an early bridge with unfractionated heparin. On POD 12 the patient was discharged from ICU to the transplant ward: a gradual favorable recovery of both the newly grafted liver and MV replaced heart went on until POD 19. Due to relapsing fever, progressive oliguria, increased bilirubinemia (up to 28 mg/dl) and worsening of respiratory function (in spite of a documented good heart function), she was readmitted to the ICU on POD 20. The neurologic function gradually deteriorated without focal signs, but with electroencephalographic (EEG) equivalents of hepatic encephalopathy West Haven grade 2: Enterococcus faecium bacteremia was documented. Worsening of liver function tests together with a sharp increase in ammonia (550 µg/dl) suggested, as a differential diagnosis, an acute rejection, excluded by a liver biopsy.
Echographic examination showed an intact hepatic vascularization. Due to a further worsening of respiratory and renal functions and deterioration of neurological conditions (agitation, and reduced consciousness, but in the complete absence of focal findings) invasive mechanical ventilation and renal replacement therapy were restarted. After a temporary good neurological and respiratory recovery, an abrupt worsening of neurologic findings (Glasgow Coma Scale 7) was evident on POD 44 with focal hyposthenia of the left arm. CT scan documented a right frontal intracranial haemorrhage. On POD 46 the patient underwent neurosurgical decompression: due to the mitral mechanical prosthetic valve, anticoagulation was not interrupted but shifted to LMWH. Unfortunately, in the next few days, the neurologic function further deteriorated, with an enlargement of the lesion and a progressive slowing of the EEG trace: the patient eventually patient died on POD 58 for brain death.
Discussion
Despite the negative outcome of this case (and in spite of that, we wish to propose the case to the attention of the scientific community), we are convinced in very selected conditions simultaneous cardiac (valve surgery in this case) and liver transplant surgery might constitute a feasible surgical option and the sole solution. The patients was successfully weaned within POD 5 from all artificial supports (ventilation, circulatory supports, CRRT). The choice of undergoing such a complex simultaneous surgical procedure with a high burden of perioperative risk could be justified only in case of relatively young patients in extreme need of OLT and/or cardiac surgery with acceptable cardiac performance status and a sufficient functional reserve in spite of the underlying cardiac pathology. As a matter of fact neither of the two interventions could have been performed alone, being considered one a contraindication to the other: cardiac surgery (MVR in this case) in ESLD patients is associated with a mortality well beyond 80%, while OLT mandates a more than acceptable cardiac reserve due to the relevant hemodynamic stress imposed by the various phases of transplant surgery [9]. Therefore, the choice of a simultaneous surgery in such a specific setting and after an appropriate thorough multidisciplinary assessment could be considered feasible: the good short term results in terms of functional recovery of both the liver graft and the heart shown in our case support our assumption. The same result was reported by Li et al. in one of the two cases reported in the literature so far. It has to be underlined that our case was performed in 2002 when no simultaneous MVR and OLT was ever reported [10, 11]. As far as we are aware of, a large part of the very few reported cases is simultaneous aortic valve replacement and OLT [8, 10, 11].
The cause of death, in this case, was a devastating intracranial haemorrhage, in a setting where the coagulopathy is frequent and the anticoagulation mandatory due to the mechanical prosthesis. A matter of hot debate is (and had been in this specific case) the choice of the mechanical prosthesis versus a bioprosthetic valve to replace the damaged valve. The critical point is (and had been) to balance the risk of a lifelong anticoagulation with a statistically longer life of the mechanical valve prosthesis (in a patient with a pre-existing coagulopathy associated with the liver disease corrected by the transplant) versus a bioprosthetic valve, in need of a much shorter anticoagulation but granted of a shorter life (10 years on average) [12], and a potential cardiac surgical procedure in medium timespan. In this case, the patient died two months after surgery, with mandatory anticoagulation therapy in any case (use of mechanical or biological prosthesis).
Was the simultaneous surgery worth? Cases of simultaneous liver transplantation and cardiac surgery are rare. In case of valve surgery, aortic valve replacement is more common (10 cases) mitral valve replacement being reported in two cases, with a survival of less than 1 year in both cases: too few cases to draw any conclusion. Li Y et al. published the case of simultaneous liver transplantation and mitral valve replacement in a 57 years-old man affected by infective endocarditis and liver cirrhosis. This patient underwent successful combined surgery but died of late, recurrent cholangitis one year after surgery [10]. Lima et al published a retrospective experience of 10 cases who underwent combined liver transplantation for cirrhosis of various etiologies and cardiac surgery for both valvular and coronary artery disease [11]. They report hospital mortality of 20% with a 3-year survival of 70 results to be considered outstanding due to such an extreme, high-risk surgery. Given the modest number of patients considered for the simultaneous intervention, no recommendations can be given. The patient we described did not die for surgical complications, but for an unavoidable side effect of necessary medication, combined with a generally compromised status due to kidney injury and a septic episode, complications quite often reported after liver surgery but not necessarily lethal.
Alternative treatment options could include percutaneous treatment of mitral valve stenosis with TMVR, followed by liver transplantation immediately or in a different moment. However, this kind of percutaneous surgery still carries a lot of technical complexities and the risks/advantages ratio is not completely clear [13-15]. As stressed in other similar experiences and in the most updated viewpoint, we can say that combined orthotopic liver transplantation and cardiac surgery is a feasible procedure in highly selected candidates: a high burden of morbidity and mortality has, nonetheless, to be accepted [15].
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Fri 27, Dec 2019Accepted: Sat 11, Jan 2020
Published: Mon 20, Jan 2020
Copyright
© 2023 Andrea De Gasperi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.1.05
Author Info
Andrea De Gasperi Elena Roselli Marcello Guarnieri
Corresponding Author
Andrea De GasperiDepartment of Anesthesia and Resuscitation 2, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy
Figures & Tables
References
- Hackl C, Schlitt HJ, Kirchner GI, Knoppke B, Loss M (2014) Liver transplantation for malignancy: current treatment strategies and future perspectives. World J Gastroenterol 20: 5331-5344. [Crossref]
- Farkas S, Hackl C, Schlitt HJ (2014) Overview of the indications and contraindications for liver transplantation. Cold Spring Harb Perspect Med 4. [Crossref]
- Yang LS, Shan LL, Saxena A, Morris DL (2014) Liver transplantation: a systematic review of long-term quality of life. Liver Int 34: 1298- 1313. [Crossref]
- Wray CL (2018) Liver Transplantation in Patients With Cardiac Disease. Semin Cardiothorac Vasc Anesth 22: 111-121. [Crossref]
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG et al. (2006) Burden of valvular heart diseases: a population-based study. Lancet 368: 1005-1011. [Crossref]
- Chandrashekhar Y, Westaby S, Narula J (2009) Mitral stenosis. Lancet 374: 1271-1283. [Crossref]
- Nishimura RA, Vahanian A, Eleid MF, Mack MJ (2016) Mitral valve disease-current management and future challenges. Lancet 387: 1324- 1334. [Crossref]
- Giakoustidis A, Cherian TP, Antoniadis N, Giakoustidis D (2011) Combined cardiac surgery and liver transplantation: three decades of worldwide results. J Gastrointestin Liver Dis 20: 415-421. [Crossref]
- Hogan BJ, Gonsalkorala E, Heneghan MA (2017) Evaluation of coronary artery disease in potential liver transplant recipients. Liver Transpl 23: 386-395. [Crossref]
- Li Y, Mederacke I, Scheumann GFW, Baraki H, Wedemeyer H et al. (2011) Simultaneous mitral valve replacement and liver transplantation. Thorac Cardiovasc Surg 59: 506-508. [Crossref]
- Lima B, Nowicki ER, Miller CM, Hashimoto K, Smedira NG et al. (2011) Outcomes of simultaneous liver transplantation and elective cardiac surgical procedures. Ann Thorac Surg 92: 1580-1584. [Crossref]
- Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL et al. (2017) Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med 377: 1847-1857. [Crossref]
- Wyler von Ballmoos MC, Kalra A, Reardon MJ (2018) Complexities of transcatheter mitral valve replacement (TMVR) and why it is not transcatheter aortic valve replacement (TAVR). Ann Cardiothorac Surg 7: 724-730. [Crossref]
- Guerrero M, Dvir D, Himbert D, Urena M, Eleid M et al. (2016) Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease With Severe Mitral Annular Calcification: Results From the First Multicenter Global Registry. JACC Cardiovasc Interv 9: 1361- 1371. [Crossref]
- Mohebali D, Anagnostopoulos A-M, Estrada-Roman A, Pavlakis M, Curry MP et al. (2019) Cardiovascular Risk Assessment in Renal and Liver Transplant Candidates: A Multidisciplinary Institutional Standardized Approach. Cardiol Rev 27: 286-292. [Crossref]
