Use of Panax Quinquefolius and Acetylcarnitine Supplement (FORT UP®) Against Fatigue in Cancer Patients Admitted to Hospice Palliative Care
A B S T R A C T
For cancer patients, fatigue is one of the most frequent and disabling symptoms. Fatigue is a mix of symptoms like muscle pain, mental cognitive dysfunction, neurological disorders that make the patient completely inactive and into a state of complete abandonment. At this time not exist anything for treatment and today there is no specific guideline; only a very few substances can alleviate this situation. In our study, we’ve used two scales of VAS to better deeply understand the severity of symptoms. For treatment, we used a supplement (Fort up® - Gamfarma Italy) made with two active principles, Panax quinquefolium and acetyl-carnitine, in 56 cancer patients admitted to hospice palliative care, administered for three weeks to assess the effect in medium to severe asthenia. Both active principles have demonstrated activity in clinical studies against cancer-related fatigue with tonic and energizing action. At the end of study 46 out of 56 patients (82%) had an improvement of symptoms and in 18 patients (4 in low, 11 in medium and 3 in severe asthenia) (32%) these symptoms there resolved. The study aimed was to investigate the efficacy of this product on cancer-related fatigue in patients admitted to hospital palliative care. Our study demonstrated that this product can relieve cancer-related fatigue in this setting of cancer patients.
Keywords
Cancer-related fatigue, cancer patients, panax quinquefolium, acetyl-carnitine palliative care
Introduction
The cancer-related fatigue (CRF) is one of the most common symptoms in cancer patients, appears frequently before cancer is diagnosed, typically increases during chemo and radiation therapy and persists after treatment. Several studies have evaluated the incidence of CRF in cancer patients: a study of 151 patients with ovarian cancer show a prevalence of fatigue in 69% of subjects, half of whom described it as highly debilitating symptoms; in another study of 913 patients who had received cancer treatment in the previous two years CRF was reported in 78% of cases and 71% of patients reported that it interfered with normal activities of daily living; a multicenter randomized trial on the treatment of lung cancer (median age 65 years ) noted fatigue and loss of energy in more than 80% of patients [1-3].
The CRF is, therefore one of the most widespread, stressful and limiting aspects related to cancer. Sometimes may even be present at the diagnosis of cancer, generally, it is a temporary side effect of treatment (especially of some chemotherapy drugs) but can last a very long time, even in people who exceed the tumour or live there for years [4]. CRF can be defined in various ways: as of today the most widely accepted definition is the one drawn up by a panel of experts belonging to the National Cancer Network Comprehensive that defines CRF as " A distressing, persistent, subjective sense of physical, emotional and cognitive tiredness or exhaustion related to cancer or cancer treatment, that is not proportional to recent activity and that interferes with usual functioning.” [5].
The mechanisms that cause or promote fatigue in patients with cancer are still poorly understood and have been the subject of several theories: CRF may be caused by many factors and the factors that contribute to cancer fatigue may be completely different from those of someone you know [6]. For measurement of fatigue, several scales unidimensional and multidimensional can be used: in our study, we have used two Scales of VAS (visual analogue scale) to better deeply understand the severity of the symptomatology. Despite the extreme gravity of the problem, currently, there are no drug treatments specifically indicated for the treatment of the CRF, except fatigue related to anaemia for which treatment with erythropoietin has shown some positive effects.
In 2015 The Cochrane Collaboration published a review of pharmacological treatments for fatigue on patients at an advanced stage of the disease, including patients with cancer and other chronic diseases: the authors included 45 randomized controlled trials, data from 18 drugs and 4696 participants: however there was a very high degree of statistical and clinical heterogeneity in the trials [7]. There was weak evidence for the efficacy of carnitine and donepezil for cancer-related fatigue, some low-quality evidence from small trials that methylphenidate is effective for the management of CRF and due to safety concerns and side effects shown by more recent studies, erythropoietin and darbepoetin should no longer be used.
The authors concluded that, based on limited evidence from small studies, the evidence does not support the use of a specific drug for the treatment of fatigue in palliative care. Very promising results have been obtained with American ginseng (Panax quinquefolium): a first randomized trial which enrolled 282 cancer patients found that American ginseng, at doses of 1,000-2,000 mg/day for 8 weeks, is effective in reducing cancer-related fatigue [8]. The same group of researchers conducted a phase III trial on 364 patients randomized to receive American ginseng at a dose of 2,000 mg/day or placebo for 8 consecutive weeks: the study has confirmed that the American ginseng, with this treatment schedule, reduces fatigue without cancer-related side effects [9].
A trial demonstrated that 1-2 grams per day of Acetyl-carnitine significantly reduce fatigue, insomnia and depression, without causing undesirable effects [10]. Based on these clinical data ARTOI ( Integrated Oncological Therapy Research Association), always committed to studying natural substances for supportive care in cancer patients, decided to test Fort Up® (GamFarma Milan Italy), a dietary supplement in 10 ml vials containing 1 gram of Panax quinquefolium titrated to 5% in ginsenosides and 1 gram of Acetyl-carnitine on the market in Italy. The aim of this study was to determine the efficacy of Fort up ® supplementation against fatigue in cancer patients admitted to hospice palliative care.
Materials and Methods
Between the 15th October 2017 to 30 April 2018, fifty-six cancer patients were recruited at the Department of Palliative Care Oncology - Villa Silvana Hospital – Aprilia (Rome), Department of Oncology – ASL Aprilia (Rome) and Outpatient Service of Integrative Oncology – Nuova Villa Claudia Hospital – Rome. Patient's characteristics: 25 female and 31male; median age 66 (range 38-76); Karnofsky performance score (KPS): 20 (8 patients) , 30 ( 22 patients) , 40 ( 16 patients) and 40-50 (10 patients); tumor type: colorectal 11 patients, lung 10 patients, brain 7 patients, gynecological 5, hematologic 4, breast 4, gastric 4, pancreatic 3, prostate 3, liver 3 and sarcoma 2 (Table 1). Informed consent was obtained from all individual participants included in the study. The inclusion criteria were as follows: the absence of anaemia, any medical treatment for fatigue (corticosteroids, carnitine, and erythropoietin) until the start of treatment.
Table 1: Patient’s characteristics.
|
Variable |
N (%) |
|
|
|
Total N= 56 |
|
|
Sex |
|
|
|
Male |
31 (55 %) |
|
|
Female |
25 (45 %) |
|
|
Karnovfsky performance status |
|
|
|
20 |
8 (14%) |
|
|
30 |
22 ( 39%) |
|
|
40 |
16 ( 29%) |
|
|
40-50 |
10 (20 %) |
|
|
Age (mean-range) |
66 (38 - 76) |
|
|
Age (mean-range) Tumor type |
66 (38 - 76) |
|
|
Colorectal cancer |
11 (20 %) |
|
|
Lung
cancer |
10 (18%) |
|
|
Brain
cancer |
7 (13 %) |
|
|
Gynecological cancer |
5 (9 %) |
|
|
Breast cancer |
4 (7 %) |
|
|
Hematologic cancer |
4 (7 %) |
|
|
Gastric cancer |
4 (7 %) |
|
|
Pancreatic cancer |
3 (5 %) |
|
|
Prostate cancer |
3 (5 %) |
|
|
Liver cancer |
3 (5 %) |
|
|
Sarcoma |
2 (4 %) |
Figure 1: Numerical fatigue measurement rating (VAS).
Figure 2: Fatigue pictogram: choose how you felt last week.
The exclusion criteria were as follows: use of medicaments for fatigue (corticosteroids, herbal medicine like Panax ginseng, astragalus, and mushrooms), inability to swallow the product. All patients were treated with 1 Fort up® vial per day at 4.00 p.m. for three weeks to assess the efficacy in cancer patients with a medium to severe fatigue. For fatigue assessment, we have used two Scales of VAS (Figures 1 & 2) to better deeply understand the severity of symptoms [11]. At baseline through the use of the scales, 10 of 56 patients have low fatigue (grade 3-4 of the scale), 31 a medium fatigue (grade 5-6 of the scale) and 15 a severity fatigue (grade 7-8 of the scale) (Table 2). The total response was when the symptoms disappeared. The partial response was when the symptoms dwindled more than 3 points. There was no response when symptoms lessened by 2 points (Figure 3).
Table 2: Grade of Fatigue at baseline.
|
Grade of fatigue |
n. patients |
|
Grade 0-4 |
10 |
|
Grade 5-6 |
31 |
|
Grade 7-10 |
15 |
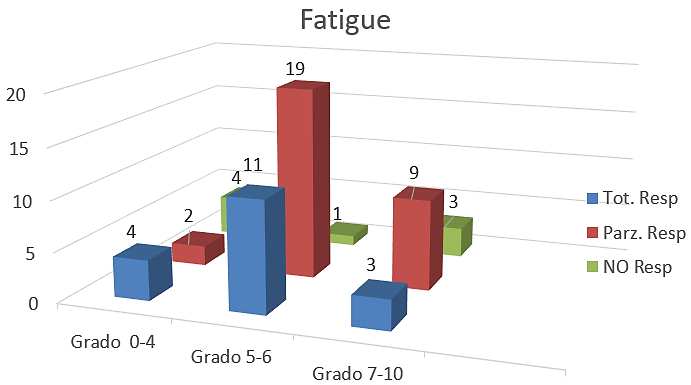
Figure 3: Number of total, partial and NO response after treatment with FORT UP.
Results
All 56 patients received 1 Fort up® vial per day for three weeks with good compliance. Analysis of VAS data showed a progressive reduction of fatigue perceived by enrolled patients during the study period. After the treatment with Fort up®, the obtained results there been very interesting: total response in 4 (four) patients with low fatigue, 11 (eleven) with medium fatigue and 3 (three) patients with severe asthenia. Partial response was in 2 (two) patients with low, 19 (nineteen) patients with medium and 9 (nine) patients with severe fatigue. No response in 4 (four) patients with low, 1 (one) patient with medium and 3 (three) patients with severe fatigue (Table 3) (Figure 3). The good result was that 46 out of 56 patients (82%) had an improvement of symptoms and in 18 patients (4 in low, 11 in medium and 3 in severe fatigue) (32%) these symptoms there resolved. Only 8 patients haven’t had a response (8%) It’s important to underline that 5 of 8 patients with KPS 20 did not respond to treatment.
Table 3: Response of fatigue at the end of study.
|
Grade of fatigue |
n. patients |
No Response |
Partial Response |
Total Response |
|
Grade 0-4 |
10 |
4 |
2 |
4 |
|
Grade 5-6 |
31 |
1 |
19 |
11 |
|
Grade 7-10 |
15 |
3 |
9 |
3 |
Discussion
The cancer-related fatigue (CRF) is one of the most common symptoms in cancer patients that heavy interfered with normal activities of daily living, even in advance disease. Analyses of cancer survivors suggest that fatigue can persist for up to 5 years after completion of treatment and possibly even longer [12]. Fatigue has a negative impact on work, social relationships, mood, and daily activi¬ties, causing significant impairment in overall quality of life during and after treatment [13]. Reports from patients suggest that cancer-related fatigue is more severe, persistent, and debilitating than ‘normal’ fatigue simply caused by lack of sleep or over¬exertion, and cancer-related fatigue is not relieved by adequate sleep or rest [14]. Cancer-related fatigue might have physical, mental, and emotional manifestations, including generalized weakness, diminished concentration or attention, decreased motivation or interest to engage in usual activities, and emotional liability [15].
Despite the prevalence and negative impact of cancer-related fatigue, this symptom is under-reported by patients, and underestimated and undertreated by clinicians: so, it is important to diagnose it early and treat it as soon as possible [16]. Currently, there are no drug treatments specifically indicated for the treatment of the CRF, except fatigue related to anaemia for which treatment with Erythropoietin has shown some positive effects. Unfortunately, psychostimulants (methylphenidate, dexamphetamine, modafinil), antidepressants (paroxetine), acetylcholinesterase inhibitors (donepezil), have reported negative results (except in some subgroup of patients with severe fatigue receiving methylphenidate and modafinil) [17]. Very promising results have been obtained with natural products, in particular, American ginseng (Panax quinquefolium): Barton’s studies underlined that the use of American ginseng can ameliorate CRF and it should be reasonable for a cancer survivor to try American ginseng for fatigue, taking into consideration that there are no other pharmacologic agents known to be effective.
A study investigating herbs for possible inhibition of the cytochrome P450 system demonstrated that American ginseng (P. quinquefolium) was one of a few herbs found to be non-inhibitory [18]. In term of safety also Barton’s paper reports that there are preclinical data demonstrating that American ginseng does not interfere with tamoxifen, doxorubicin, cyclophosphamide, paclitaxel, 5-fluorouracil, and methotrexate. Attention should be paid to the type of ginseng purchased considering that different types of ginseng, with different amounts, strengths, and varieties of ginsenosides, are available on the market [9]. In fact, a double-blind randomized study conducted on 127 patients demonstrated that Panax ginseng, at the dosage of 400 mg twice daily, was not significantly superior to placebo in fatigue treatment after 4 weeks of treatment and based on this there is no justification to recommend the use of Panax ginseng for CRF [19]. Our study confirmed that using Panax quinquefolium (Fort up®) induces a progressive reduction of fatigue perceived by enrolled patients.
Conclusions
The fatigue is a very important problem for cancer patients and heavy interfered with normal activities of daily living: it is important to diagnose it early and treat it as soon as possible. Currently, there are no drug treatments specifically indicated for the treatment of cancer- related fatigue. In our study Fort up® (a dietary supplement containing Panax quinquefolium and Acetyl-carnitine) obtained very interesting results in the treatment of fatigue a palliative setting: 82% of patients had an improvement of symptoms and in 32% of them these symptoms there resolved. Only the patients with very low KPS did not have response to treatment. Logically the sample of the study is small for any statistical analysis and should be taken as a point of reference for a larger confirmatory study.
Acknowledgement
Thanks to Roberto Galante and Fabrizio Mira of Gamfarma for the free donation of FORT UP.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 09, Apr 2020Accepted: Wed 22, Apr 2020
Published: Thu 30, Apr 2020
Copyright
© 2023 Massimo Bonucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.04.10
Author Info
S. Dell’Arte A. Siniscalchi G. Spinelli Massimo Bonucci
Corresponding Author
Massimo BonucciDepartment of Outpatient Oncology, Villa Benedetta Hospital, Rome, Italy
Figures & Tables
Table 1: Patient’s characteristics.
|
Variable |
N (%) |
|
|
|
Total N= 56 |
|
|
Sex |
|
|
|
Male |
31 (55 %) |
|
|
Female |
25 (45 %) |
|
|
Karnovfsky performance status |
|
|
|
20 |
8 (14%) |
|
|
30 |
22 ( 39%) |
|
|
40 |
16 ( 29%) |
|
|
40-50 |
10 (20 %) |
|
|
Age (mean-range) |
66 (38 - 76) |
|
|
Age (mean-range) Tumor type |
66 (38 - 76) |
|
|
Colorectal cancer |
11 (20 %) |
|
|
Lung
cancer |
10 (18%) |
|
|
Brain
cancer |
7 (13 %) |
|
|
Gynecological cancer |
5 (9 %) |
|
|
Breast cancer |
4 (7 %) |
|
|
Hematologic cancer |
4 (7 %) |
|
|
Gastric cancer |
4 (7 %) |
|
|
Pancreatic cancer |
3 (5 %) |
|
|
Prostate cancer |
3 (5 %) |
|
|
Liver cancer |
3 (5 %) |
|
|
Sarcoma |
2 (4 %) |
Table 2: Grade of Fatigue at baseline.
|
Grade of fatigue |
n. patients |
|
Grade 0-4 |
10 |
|
Grade 5-6 |
31 |
|
Grade 7-10 |
15 |
Table 3: Response of fatigue at the end of study.
|
Grade of fatigue |
n. patients |
No Response |
Partial Response |
Total Response |
|
Grade 0-4 |
10 |
4 |
2 |
4 |
|
Grade 5-6 |
31 |
1 |
19 |
11 |
|
Grade 7-10 |
15 |
3 |
9 |
3 |
References
- Portenoy RK, Itri LM (1999) Cancer-related fatigue: guidelines for evaluation and management. Oncologist 4: 1-10. [Crossref]
- Ashbury FD, Findlay H, Reynolds B, McKerracher K et al. (1998) A Canadian survey of cancer patients experience: are their needs being met? J Pain Symptom Manage 16: 298-306. [Crossref]
- Hopwood P, Stephens RJ (1995) Symptoms at presentation for treatment in patients with lung cancer: implication for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer 71: 633-636. [Crossref]
- Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL et al. (2005) Long-Term Health-Related Quality of Life, Growth, and Spiritual Well-Being after Hematopoietic Stem-Cell Transplantation. J Clin Oncol 23: 599-608. [Crossref]
- NCCN Fatigue Practice Guidelines Panel version 1: 2014.
- Gutstein HB (2001) The biologic basis of fatigue. Cancer 92: 1678-1683. [Crossref]
- Mücke M, Mochamat, Cuhls H, Peuckmann-Post V, Minton O et al. (2015) Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev: CD006788. [Crossref]
- Barton DL, Soori GS, Bauer BA, Sloan JA, Johnson PA et al. (2010) Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer 18: 179-187. [Crossref]
- Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA et al. (2013) Wisconsin Ginseng (Panax quinquefolius) to Improve Cancer-Related Fatigue: A Randomized, Double-Blind Trial, N07C2. J Natl Cancer Inst 105: 1230-1238. [Crossref]
- Cruciani RA, Dvorkin E, Homel P, Malamud S, Culliney B et al. (2006) Safety, Tolerability and Symptom Outcomes associated with L-Carnitine Supplementation in Patients with Cancer, Fatigue, and Carnitine Deficiency: A Phase I/II Study. J Pain Symptom Manage 32: 551-559. [Crossref]
- Maxwell C (1978) Sensitivity and accuracy of the visual analogue scale: a psycho-physical classroom experiment. Br J Clin Pharmacol 6: 15-24. [Crossref]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH et al. (2006) Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 106: 751-758. [Crossref]
- Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ et al. (2000) Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist 5: 353-360. [Crossref]
- Poulson MJ (2001) Not just tired. J Clin Oncol 19: 4180-4181. [Crossref]
- Cella D, Davis K, Breitbart W, Curt G, Fatigue Coalition (2001) Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 19: 3385-3391. [Crossref]
- Mitchell SA (2010) Cancer-related fatigue: state of the science. PM R 2: 364-383. [Crossref]
- Roila F: Il trattamento farmacologico della fatigue da cancro CASCO 2015.
- Budzinski JW, Foster BC, Vandenhoek S, Arnason JT et al. (2000) An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 7: 273-282. [Crossref]
- Yennurajalingam S, Tannir NM, Williams JL, Lu Z, Hess KR et al. (2017) A Double-Blind, Randomized, Placebo-Controlled Trial of Panax Ginseng for Cancer-Related Fatigue in Patients With Advanced Cancer. J Natl Compr Canc Netw 15: 1111-1120. [Crossref]
