Unilateral 6-Hydroxydopamine-Lesioned Rat as Relevant Model to Study the Pain Related to Parkinson’s Disease
A B S T R A C T
The present study was conducted to test the use of the hemiparkinsonian rat, obtained by the unilateral injection of 6-hydroxydopamine (6-OHDA) in the substantia nigra pars compacta (SNc), as a suitable model for the study of pain associated to Parkinson’s disease (PD). For this purpose, 14 days after unilateral injection of saline or 6-OHDA, rats were assessed for behavioral function in the cylinder test, and apomorphine-induced circling test. Thereafter, at 21st day after injection, mechanical nociceptive threshold was compared between 6-OHD-lesioned and sham-operated animals using electronic von Frey test. Our results showed that injection of 6-OHDA in the SNc induced alterations of behavioral motor as ascertained by predominant use of the ipsilateral forepaw in cylinder test and by the expression of contralateral turnings after subcutaneous injection of apomorphine. The mechanical nociceptive threshold was significantly decreased in 6-OHDA-lesioned rats compared to that of sham-operated rats (p <0.05). This response was reversed by apomorphine treatment. In conclusion, hemiparkinsonian rat, obtained by the unilateral injection of the 6-OHDA in the SNc, can be used to investigate pain symptoms and central pain processing mechanisms related to PD.
Keywords
6-hydroxydopamine, dopaminergic pathway, nociception, pain, Parkinson disease, behavior
Introduction
Parkinson’s disease (PD) is a progressive and incurable neurodegenerative disorder affecting and recognized clinically by its motor symptoms, such as bradykinesia, rest tremor, rigidity, and postural instability [1]. It is a multifactorial neurodegenerative disease characterized by region-specific loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) [2]. In recent years, non-motor symptoms in PD patients, including neuropsychiatric symptoms, gastrointestinal symptoms, sexual difficulties, sleep disorders, and pain have been well characterized. Pain is the most prominent non-motor symptom observed in patients with PD. About 40-60 % of patients with PD experience various types of acute or chronic pain [3].
Pain has a negative impact on the quality of life in patients with PD and, in a small subset of patients; pain can actually be the dominant symptom of PD [4]. Chronic pain remains under recognized and poorly treated in PD patients. Pain can be related to motor symptoms of PD (muscle cramps, painful dystonias, or diphasic dyskinesias) but primary sensory complaints unrelated to motor disability have also been described [5]. In most PD cases, pain appears before motor and cognitive symptoms and is reported by at least 33 % of patients [6]. Despite the high incidence of pain in PD patients, few studies have explored the relationship between nigrostriatal injury and pain circuitry. It is well known that degeneration of dopaminergic neurons in the SNc, where the major dopaminergic neurons projected to the motor-regulating nucleus in the basal ganglia, contributes to the motor symptoms and pain in PD patients [7-9].
To date, the exact mechanism of pain perception in PD is still elusive, for this reason, it is necessary to define the pathophysiology in rodent models of PD. Unilateral 6-hydroxydopamine (6-OHDA)-induced lesion of the nigrostriatal pathway in rats is widely used as a PD model. However, in the few experiments where this model was used to study nociception, the injection of 6-OHDA was often done in the striatum [10, 11]. Intranigral injection of 6-OHDA has emerged as an interesting third alternative. This version, which was introduced by Parish et al., is attractive in that it makes it possible to induce more extensive dopamine neurodegeneration in the absence of the high mortality rate seen in medial forebrain bundle and striatum-lesioned animals [12]. Then we used, in the present study, unilateral 6-OHDA, obtained by the injection of the neurotoxin directly in SNc, in order to test its relevance as a model for the study of pain related to PD.
Material and Methods
I Animals
Three-month-old male Wistar rat (220-280 g) were used in the present study. The animals were housed according to the EEC 609/86 Directives regulating the welfare of experimental animals and experiments were approved by the local ethics committee of Institute of Biotechnology (University of Monastir, Tunisia). Animals were maintained in individual stainless-steel cages under standard conditions of controlled photoperiod (12:12 h light/dark schedule) and temperature (22±2 ◦C). They had access to a standard rodent laboratory diet and drinking water ad libitum.
II Surgical Procedure
Rats were anaesthetized by 4% chloral hydrate (i.p. 10 mL/kg). They were placed in a stereotaxic apparatus with the incisor bar positioned 3.3 mm below the interaural line. To achieve lesioning of the nigrostriatal pathway, 4 μL of 6-OHDA (2 μg/μL free based in 0.2 % ascorbic acid dissolved in saline preventing heat and light exposure) was stereotactically injected unilaterally into the SNc (anteroposterior: –5.2, mediolateral: +2.1 and dorsoventral: –7.6mm from bregma; Paxinos and Watson, 2007) using a 5-μL Hamilton Syringe (Sigma, St Louis, MO, USA) [13]. Syringe was lowered into the brain at a rate of 2 mm/min, 6-OHDA was injected at a rate of 1 μL/min, and the syringe was left in place for 5 min after injection before being drawn back at a rate of 2 mm/min [14]. Sham-operated animals (n=6) underwent the same surgical procedure of the 6-OHDA rats with the injection of an equal volume of the vehicle only. After surgery, all animals were individually housed and monitored for the time of recovery from anesthesia.
III Behavioral Tests
i Cylinder Test
Forelimb use was assessed using the cylinder test, as previously described by Schallert et al. [15]. The protocol was performed 14 days after the injection of 6-OHDA. Rats were placed for 10 min in a glass cylinder (diameter 19 cm, height 20 cm), with mirrors placed behind to allow for a 360 view of all touches. The number of touches on the cylinder wall with single right and left forepaw during their standing on rear limbs was determined for 6-OHD-lesioned and sham-operated animals. Only animals with statistically significant preference for the left forepaw were counted among animals with Parkinsonism and included in group 6-OHDA.
ii Apomorphine-Induced Circling
6-OHDA-lesioned or sham-operated rats were tested in automated rotameter cylinders (Panlab, Barcelona, Spain). The turning behavior (defined as a full 360°rotation of the body axes) was measured after apomorphine hydrochloride s.c. injection (0.2 mg/kg free based, Sigma-Aldrich). The total number of net rotations (contralateral minus ipsilateral rotations) was recorded for 40 min. Lesioned animals that did not reach a cut-off value of .5 contralateral turns, per minute, indicative of 70% nigral cell loss, were excluded from further analysis [16].
IV von Frey Test for Mechanical Allodynia
Mechanical allodynia was assessed as described by Thibault et al. [17]. Rats were individually placed on an elevated wire mesh floor in a clear plastic box and were adapted to the testing environment for 10 min. An Electronic von Frey unit (EVF-4, Bioseb, Chaville, France) was used: the sensitivity threshold is measured in one test, measurement ranging from 0.1 to 100 g with a 0.2 g accuracy. Punctuate stimulus is delivered to the mid-plantar area of each hind paw from below the mesh floor through a plastic spring tip and sensibility threshold result is displayed on a screen. Paw sensitivity threshold was defined as the minimum pressure required eliciting a robust and immediate withdrawal reflex of the paw. Voluntary movement associated with locomotion was not taken as a withdrawal response. The stimulus was applied on each hind paw five times with a five seconds interval and the value adopted as a threshold for a rat was the average of the ten values measured. Mechanical allodynia was defined as a significant decrease in withdrawal thresholds to EVF-4 application (Thibault et al. 2008) [17]. To verify whether nigrostriatal dopaminergic pathway is implicated in pain processing, we performed the restoration experiments with apomorphine. For this reason, mechanical allodynia was also assessed after 5 min of subcutaneously injection of apomorphine hydrochloride (0.2 mg/kg free based) or saline [18].
V Statistical Analysis
Data were shown as mean ± standard error of the mean (SEM). Differences were considered significant when p<0.05. Statistical analysis was completed by one-way ANOVA, followed by Tukey’s post hoc t-test where appropriate with Prism 5.0, GraphPad Software (San Diego, CA, USA).
Results
I Assessment of Nigrostrial Lesion
i Cylinder Test
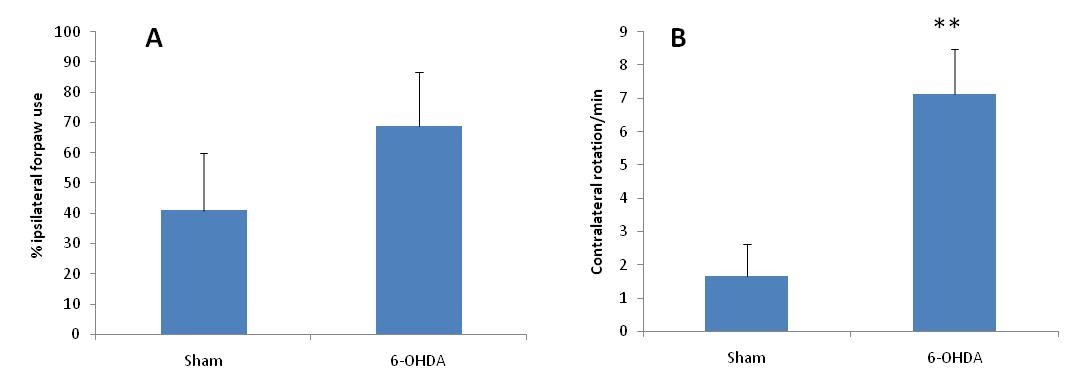
The administration of 6-OHDA was effective to cause nigrostrial lesion to the left hemisphere as ascertained by predominant use of the ipsilateral forepaw, which was determined by the increase (but not significant) number of touches of this limb on the cylinder wall as compared with the right one, 14 days after the injection of this drug (Figure 1A). The injection of saline (sham group) produced no changes in expected normal use of forelimbs.
Figure 1: A) Cylinder test showed an increase (but not significant) number of touches of the ipsilateral limb (toward th elesion site) on the cylinder wall in 6-OHDA exposed animals (n=8) as compared with sham-injured animals (n=6). B) Apomorphine-circling test showed a significant increase (p < 0.01) in contralateral rotations of 6-OHDA-exposed animals (n = 8) as compared with sham-injured animals (n=6).
** : p < 0.01 compared to the sham-operated animals.
ii Apomorphine-Induced Circling
Unilateral 6-OHDA injection was followed by a rotametre test after 2 weeks (Figure 1B). Compensation of dopamine depletion with apomorphine led to a significant rotational response in injured rats which showed rotational scores > 5 net full turns/min in the contralateral direction to the lesion. These rotational scores were found to correspond to > 70% depletion of dopaminergic neurons.
II Effect of Nigrostrial Lesion on Body Weight Gain
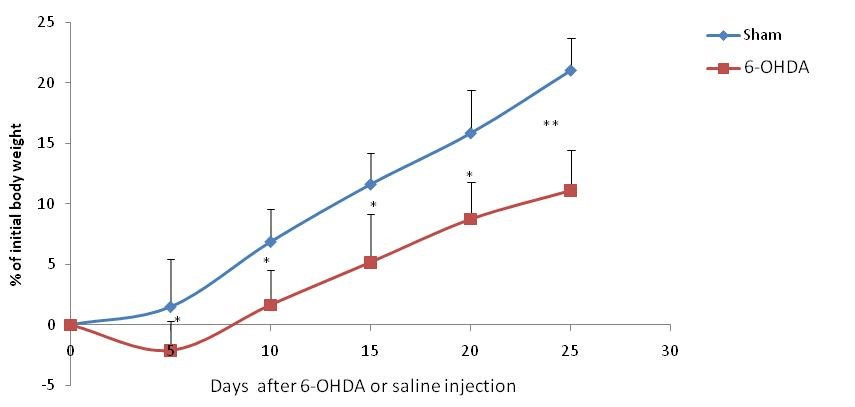
After the fifth day of the injury, the body weight gain of 6-OHDA-lesioned and sham-operated rats increased during the experiment (Figure 2), but weight gain was significantly more important in the shame-operated group during the four weeks after the lesion. No animals died during the experiment.
Figure 2: Gain body weight expressed as % initial body weight after injection of 6-OHDA (6-OHDA- exposed rats, n=8) or saline (Sham-operated rats, n=6).
* : p < 0.05 compared to the sham-operated animals in the same day.
** : p < 0.01 compared to the sham-operated animals in the same day.
III Effect of Nigrostrial Lesion on Mechanical Nociceptive Thresholds
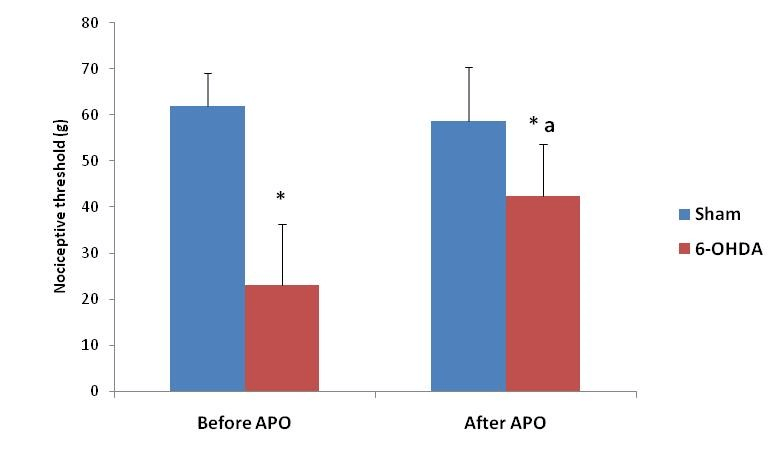
As measured with the EVF-4 (Figure 3), the mean withdrawal reflex of the paw was significantly reduced in 6-OHDA-lesioned rats (23.09±13.23 g at the 21th day after lesion versus 61.91±07.26 g) (P≤0.05) when compared with sham-operated rats. The decrease in withdrawal thresholds in 6-OHD-treated rats was fully abrogated by apomorphine administration (Figure 3). In fact, 6-OHDA-lesioned rats treated by apomorphine showed significantly increased response latency in the von Frey test compared to their response before apomorphine injection (42.42±11.32 g versus 23.09±13.23 g).
Figure 3: Mechanical nociceptive threshold in 6-OHDA exposed animals (n=8) as compared with sham-injured animals (n=6), before and after subcutaneously injection of apomorphine (APO).
* : p < 0.05 compared to the sham-operated animals.
a : p < 0.05 compared to the 6-exposed animals before apomorphine injection.
Discussion
Since pain is an important nonmotor symptom in PD, a better understanding of its pathophysiological mechanisms can lead to treatment and quality of life optimization in these patients. The choice of an adequate animal model is crucial to elucidate the mechanisms involved in this pain. The present study was conducted to test the use of the hemiparkinsonian rat, obtained by the unilateral injection of the 6- 6-OHDA in the SNc, as a suitable model for the study of pain associated to PD. Systemically administered 6-OHDA fails to cross the blood–brain barrier. Thus, 6-OHDA has to be injected stereotactically into the brain. Preferred injection sites are the SNc, medial forebrain bundle, and striatum [19, 20]. Although generally the nerve terminals are more sensitive to 6-OHDA toxicity than the axon and cell body, when injected into the SNc or the medial forebrain bundle, 6-OHDA produces a complete and rapid lesion in the nigrostriatal pathway [21]. When injected into the SNc, degeneration of dopaminergic neurons takes place within 12 h preceding a significant loss of striatal terminals, which occurs 2–3 days later [22]. The degree of damage of dopaminergic neurons in the nigrostriatal pathway obtained with 6-OHDA injection in striatum is less marked compared to the SNc or intra-medial forebrain bundle injection, remaining confined to 50–70% of the nucleus, and evolves over a period of 4–6 weeks [23]. For all these reasons we have chosen, in the present study, to inject 6-OHDA into SNc.
The major advantage of using the 6-OHDA model is its rather unique effect on quantifiable motor abnormality in animals which allow estimating the percentage of degeneration of dopaminergic neurons. Our results showed that depletion of dopaminergic neurons in the nigrostriatal pathway by the unilateral injection of the 6-OHDA in the SNc induced an alteration of behavioral motor as ascertained by predominant use of the left forepaw in cylinder test and by the expression of contralateral turnings after systemic injections of apomorphine; which is a dopamine receptor agonist stimulating both classes of dopamine receptors (D1, D2). This contralateral response is attributed to the stimulation of supersensitive D1-receptor and D2-receptor activation, especially in the lesioned hemisphere [24]. The decrease of the body weight gain described in 6-OHD-lesionned rat can be explained by the toxic effects of the neurotoxin. In fact, once taken up into neurons, 6-OHDA accumulates in the cytosol where it is readily oxidized leading to the generation of reactive oxygen species and ultimately, oxidative stress-related cytotoxicity [25].
6-OHDA-injected rats exhibited more sensitive nociceptive behavior when mechanical stimulation was introduced. 6-OHDA-injected rats showed shorter paw withdrawal latencies in the automated von Frey test compared to sham-operated rats. This indicated that hypersensitivity to mechanical stimuli was elicited by 6-OHDA injection. Consistent with our results, mesostriatal 6- OHDA administration induced mechanical allodynia in the paw ipsilatéral to the nigrostriatal lesion 35 days after PD model induction [11]. A similar effect was also observed with acute intraperitoneal injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice [18]. The dopaminergic system in the striatum has been implicated in pain. Inhibition or activation of striatal dopamine D2 receptors is related to nociceptive responses [26]. Human studies also support the function of dopamine D2 receptors in pain [27].
These results suggest that the dopaminergic nigrostriatal pathway also has a role in inhibiting basal mechanical nociception. Since dopaminergic neurons are involved in nociceptive response modulation, inhibition of SNc activation after striatal 6-OHDA injection can directly contribute to the decreased nociceptive threshold observed in the present study [8, 26, 28]. The nociceptive threshold reduction was reversed by systemic administration of dopaminergic agonist, apomorphine, which is consistent with previous data showing that dopamine replacement improves pain behavior in PD patients [29, 30]. In conclusion, our data showed that 6-OHDA injected unilaterally in SNc of rat induces mechanical hypernociception, which was reversed by apomorphine as a dopamine receptor agonist. Thus, hemiparkinsonian rat obtained by the unilateral injection of the 6-OHDA in the SNc, can be used to investigate pain symptoms and central pain processing mechanisms related to PD.
Acknowledgements
Authors thank Miss Anwar Hadj Hamza, technician of the animal facility, for her availability and her very precious help.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 19, Dec 2019Accepted: Mon 06, Jan 2020
Published: Fri 17, Jan 2020
Copyright
© 2023 Imed Messaoudi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2019.04.03
Author Info
Ameni Nsibi Hana Saoud Imed Messaoudi Nour Elhouda Saidi Sahar Mani
Corresponding Author
Imed MessaoudiInstitute of Biotechnology, University of Monastir, Tunisia
Figures & Tables
** : p < 0.01 compared to the sham-operated animals.
* : p < 0.05 compared to the sham-operated animals in the same day.
** : p < 0.01 compared to the sham-operated animals in the same day.
* : p < 0.05 compared to the sham-operated animals.
a : p < 0.05 compared to the 6-exposed animals before apomorphine injection.
References
- Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386: 896-912. [Crossref]
- Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373: 2055-2066. [Crossref]
- Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5: 235-245. [Crossref]
- Wasner G, Deuschl G (2012) Pains in Parkinson disease--many syndromes under one umbrella. Nat Rev Neurol 8: 284-294. [Crossref]
- Snider SR, Fahn S, Isgreen WP, Cote LJ (1976) Primary sensory symptoms in Parkinsonism. Neurology 26: 423- 429. [Crossref]
- Beiske AG, Loge JH, Rønningen A, Svensson E (2009) Pain in Parkinson's disease: prevalence and characteristics. Pain 141: 173-177. [Crossref]
- DeLong MR, Wichmann T (2015) Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurology 72: 1354-1360. [Crossref]
- Chudler EH, Dong WK (1995) The role of the basal ganglia in nociception and pain. Pain 60: 3-38. [Crossref]
- Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8: 464-474. [Crossref]
- Tassorelli C, Armentero MT, Greco R, Fancellu R, Sandrini G et al. (2007) Behavioral responses and Fos activation following painful stimuli in a rodent model of Parkinson's disease. Brain Res 1176: 1153-1161. [Crossref]
- Takeda R, Ishida Y, Ebihara K, Abe H, Matsuo H et al. (2014) Intrastriatal grafts of fetal ventral mesencephalon improve allodynia-like withdrawal response to mechanical stimulation in a rat model of Parkinson's disease. Neurosci Lett 573: 19-23. [Crossref]
- Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK (2001) The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci 21: 5147-5157. [Crossref]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. 7th ed. Amsterdam: Elsevier Academic Press.
- De Jesús-Cortés H, Miller AD, Britt JK, DeMarco AJ, De Jesús-Cortés M et al. (2015) Protective efficacy of P7C3-S243 in the 6-hydroxydopamine model of Parkinson’s disease. NPJ Parkinsons Dis 1: 15010. [Crossref]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39: 777-787. [Crossref]
- Zhang X, Andren PE, Greengard P, Svenningsson P (2008) Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci U S A 105: 2163-2168. [Crossref]
- Thibault K, Elisabeth B, Sophie D, Claude FZ, Bernard R et al. (2008) Antinociceptive and anti-allodynic effects of oral PL37, a complete inhibitor of enkephalin-catabolizing enzymes, in a rat model of peripheral neuropathic pain induced by vincristine. Eur J Pharmacol 600: 71-77. [Crossref]
- Park J, Lim CS, Seo H, Park CA, Zhuo M et al. (2015) Pain perception in acute model mice of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mol Pain 17: 11-28. [Crossref]
- Perese DA, Ulman J, Viola J, Ewing SE, Bankiewicz KS (1989) A 6-hydroxydopamine-induced selective parkinsonian rat model. Brain Res 494: 285-293. [Crossref]
- Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson Lewis V et al. (1995) Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 67: 631-647. [Crossref]
- Jonsson G (1983) Chemical lesioning techniques: Monoamine neurotoxins. In Handbook of chemical neuroanatomy Vol 1: Methods in chemical neuroanatomy (ed. Bjo¨rklund A, Ho¨kfelt T). Elsevier, Amsterdam 463-507.
- Jeon BS, Jackson Lewis V, Burke RE (1995) 6-hydroxydopamine lesion of the rat substantia nigra: Time course and morphology of cell death. Neurodegeneration 4: 131-137. [Crossref]
- Blandini F, Armentero MT (2012) Animal models of Parkinson’s disease. FEBS J 279: 1156-1166. [Crossref]
- Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318: 215-224. [Crossref]
- Tieu K (2011) A Guide to Neurotoxic Animal Models of Parkinson’s Disease. Cold Spring Harb Perspect Med 1: a009316. [Crossref]
- Magnusson JE, Fisher K (2000) The involvement of dopamine in nociception: the role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res 855: 260-266. [Crossref]
- Hagelberg N, Jääskeläinen SK, Martikainen IK, Mansikka H, Forssell H, et al. (2004) Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol 500: 187-192. [Crossref]
- Taylor AMW, Becker S, Schweinhardt P, Cahill C (2016) Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157: 1194-1198. [Crossref]
- Gerdelat Mas A, Simonetta Moreau M, Thalamas C, Ory Magne F, Slaoui T et al. (2007) Levodopa raises objective pain threshold in Parkinson's disease: a RIII reflex study. J Neurol Neurosurg Psychiatry 78: 1140-1142. [Crossref]
- Dellapina E, Gerdelat Mas A, Ory Magne F, Pourcel L, Galitzky M et al. (2011) Apomorphine effect on pain threshold in Parkinson's disease: a clinical and positron emission tomography study. Mov Disord 26: 153-157. [Crossref]
