Ultrasonic Activation of the Sonosensitizer Fimaporfin Enhances the Efficacy of Chemotherapy: An In Vitro Study on Rat Glioma Cells
A B S T R A C T
Activation of sonosensitizers via focused ultrasound, i.e., sonodynamic therapy, has been proposed as an alternative to light-activated photodynamic therapy for the treatment of a number of conditions from cancer to bacterial infections. The use of focused ultrasound allows treatment to sites buried deep within tissues, overcoming one of the main limitations of light-based modalities. Photochemical internalization is a technique that utilizes the photochemical properties of photodynamic therapy for the release of trapped endo-lysosomal macromolecules into the cell cytoplasm, greatly enhancing their efficacy. We have examined ultrasonic activation of disulfonated tetraphenyl chlorin (fimaporfin) together with the anti-cancer agent bleomycin, termed sonochemical internalization, as an alternative to light-activated photochemical internalization. Our results indicate that, compared to drug or focused ultrasound treatment alone, focused ultrasound activation of fimaporfin together with BLM significantly inhibits the viability of glioma monolayers and the treated cells’ ability to form clonogenic colonies.
Keywords
Fimaporfin, sonochemical internalization, sonodynamic therapy, glioma cell line, bleomycin
Introduction
An important limitation to chemotherapy for cancer is that drugs must gain entry into cells through the plasma membrane, limiting chemotherapeutic agents to mostly lipophilic or low molecular weight compounds that passively diffuse into the cell cytoplasm. In contrast, many highly effective chemotherapeutic agents are large and water soluble and are actively transported into cells by endocytosis. Their poor ability to escape from the resulting intracellular endosomes leads to their inactivation via lysosome-endosome fusion. Photochemical internalization (PCI) is a method that leads to increased endosomal escape and significantly increases the efficacy of a large variety of agents [1-5]. PCI requires the use of specific membrane localizing photosensitizers that are transported into the cell via adsorptive endocytosis during which, the photosensitizer remains in the endosome membrane while the macromolecule is confined to the lumen.
The vast majority of PCI studies have employed one of two amphiphilic photosensitizers aluminium phthalocyanine disulfonate (AlPcS2a) or meso-tetraphenyl porphyrin disulfonate (TPPS2a) which localize in the membranes of endosomes and lysosomes and, upon light activation, interacts with ambient oxygen yielding singlet molecular oxygen, a potent reactive oxygen species. The short lifetime of singlet oxygen in biological tissues limits its diffusion length (< 20 nm), and as such, damage to the vesicular membrane will be confined to the immediate vicinity of the photosensitizer. Upon release from the damaged endosome, the therapeutic macromolecule is able to diffuse through the cytosol to its intended target (e.g., DNA in the case of chemotherapeutic agents) instead of being transported and degraded in lysosomes.
We have previously shown in vitro, that in the presence of the sonosensitizer, AlPcS2a, focused ultrasound (FUS) can significantly enhance the effectiveness of chemotherapy by enabling endosomal escape [6-8]. This technique, termed sonochemical internalization (SCI), is comparable to light-based PCI but without the intrinsic drawback of limited penetration depth of light in biological tissues. The basic SCI concept is illustrated in (Figure 1).
Figure 1: Illustration of SCI of BLM. (a) 1) BLM enters the cell via endocytosis, 2) lysosome-endosome fusion 3) drug is trapped and degraded and never reaches its target. (b) Cell membrane is loaded with sonosensitizer 1) BLM enters the cell together with the sonosensitizer via endocytosis; the sonosensitizer and the drug co-localize in the endosome, with the sonosensitizer localized in the membrane and the drug in the lumen. 2). Application of FUS results in endosome rupture. 3) Escape of drug into the cytosol and subsequent interaction with its intended target.
The synergistic effect of SCI, employing relatively low drug concentrations and FUS power levels, has the potential to reduce the problems of nonspecific tissue damage caused by thermal effects at high ultrasonic power and unwanted drug morbidity. Unfortunately, the use of AlPcS2a as the photo/sono sensitizer has proven unsuitable for standard clinical use and presently lacks clinical approval. The photosensitizer disulfonated tetraphenyl chlorin (TPCS2a; fimaporfin), with a smaller number of isomers, and lower batch-to-batch variation compared to AlPcS2a was, therefore, developed [9-10]. Fimaporfin has been used in several clinical studies, among these the first clinical trial of PCI with the drug bleomycin [11]. The purpose of the study reported here was to evaluate the increased efficacy of bleomycin (BLM) by fimaporfin mediated SCI. An in vitro model employing rat glioma monolayer cultures was used to evaluate SCI efficacy via clonogenicity and viability assays.
Materials and Methods
I Cell Lines
The F98 rat glioma cell line was obtained from ATCC (CRL-2397) and was used in all cell monolayer experiments. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Carlsbad, California, USA) with high glucose and supplemented with 2 mM L-glutamine, gentamycin (100 mg/ml), and 2% heat-inactivated fetal bovine serum (Gibco). Cells were maintained at 370C in a 7.5% CO2 incubator.
II Sonosensitizer/Drugs
TPCS2a (the Amphinex formulation) was obtained from, PCI Biotech AS, Oslo, Norway. Bleomycin, (BLM) was obtained from Sigma, St. Louis, Missouri, USA.
III Fluorescence Microscopy of TPCS2a Intra-Cellular Distribution
1 × 10^4 F98 cells were plated out in 35 mm glass-bottomed imaging dishes (Fluorodish Cell Culture Dish, Florida, USA) and incubated in culture medium for 24 h to allow them to adhere. The F98 cells were incubated with 1 μg/ml TPCS2a for 18 h, followed by a triple wash and a 4 h incubation in the pure medium. FUS irradiation was performed with a portable FUS generator (SonoCare Plus, Roscoe Medical, Inc., Strongsville, Ohio, USA) at 0.2 W/cm2 for 1 min (1 MHz, CW). One hour after FUS irradiation, fluorescence microscopy was done, and the cells were visualized using an inverted Zeiss laser-scanning microscope (LSM 410, Carl Zeiss, Jena, Germany).
IV Glioma Cell Growth Viability Assays
Two techniques were used for treatment and for assessing the results of the various treatment parameters. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS, Promega, Madison, Wisconsin, USA), was used to determine cell viability after treatment. Six wells (in 6-well flat-bottomed plates) for each set of treatment conditions were seeded with F98 cells at a density of 15,000 cells per well and incubated for 24 h prior to experimentation. The cells were exposed to either of the following: no treatment, BLM, BLM+FUS, TPCS2a +FUS (SDT) or TPCS2a + BLM +FUS (SCI). Following treatment, incubation was continued for 48 h at which point the culture medium was replaced with fresh clear buffer containing MTS reagents and incubated for a further 2 h. The optical density was read using an ELx800uv Universal Microplate Reader (BIO-TEK Instruments, Inc.).
Long-term survival was studied using a clonogenicity assay. F98 cells were plated out in 6 well tissue culture plates at 100 cells per well and incubated overnight to allow them to adhere. The treatment groups were the same as for the MTS assay. Following treatment, the cells were allowed to grow for an additional 14 d whereupon they were washed, fixed in ethanol and stained with 0.5% crystal violet in 95% ethanol. Colonies containing more than 50 cells were scored as survivors. The colonies were photographed, and their number was counted visually. The number of colonies was normalized to a control group that received no treatment.
V BLM and BLM +FUS Toxicity
The viability of treated cells was assayed as described in the previous section. The cultures received either BLM only or BLM + FUS. Varying concentrations (0.075-0.3 μg/ml) of BLM in culture medium was added to the cultures. FUS irradiation was performed as previously described. The cultures received a range of US exposures (0-0.2 W/cm2) delivered over an interval of 1 or 2 min. The cells were allowed to grow for an additional 48 h (MTS assay) or 11-14 d (clonogenic assay). All experiments were performed in duplicate, and results are an average of at least four separate experiments.
VI SDT/SCI
The viability of treated cells was assayed as described previously. Following an initial 24 h incubation, cells were administered 0.3 μg/ml TPCS2a in DMEM for 18 h. For SDT evaluation, the cells were washed three times with PBS, and 1 ml of fresh medium was added, and incubation was continued for a 4 h “soak” period followed by FUS irradiation performed as previously described. The cultures received a range of US exposures (0-0.4 W/cm2) delivered over an interval of 1 or 2 min. For SCI evaluation, following a triple wash, BLM was added at various concentrations (0.075-0.3 μg/ml) and incubation was continued for a 4 h “soak” period followed by FUS irradiation (0-0.2 W/cm2) for 1 or 2 min. In both cases, the cells were allowed to grow for an additional 48 h (MTS assay) or 11-14 d (clonogenic assay). All experiments were performed in duplicate, and results are an average of at least four separate experiments.
VII Statistical Analysis
All data were analyzed and graphed using Microsoft Excel. The arithmetic mean and standard error were used throughout to calculate averages and errors. Statistical significance was calculated using the Student’s t-test as well as the Welch’s t-test. Two values were considered distinct when their p-values were below 0.05.
Synergism was calculated when analyzing SCI treatments. The following equation was used to determine if the SCI effect was synergistic, antagonistic, or additive [17]:
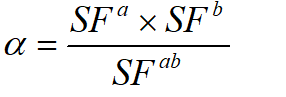
In this scheme, SF represents the survival fraction for a specific treatment. If two treatments are to be compared, the survival fractions of each separate treatment are multiplied together and then divided by the survival fraction when both treatments are applied together. The resulting parameter (α) describes the cumulative effect. If α > 1, the result is synergistic. If α <1, the result is antagonistic, and if α = 1, the result is simply additive.
Results
I Endosomal Escape of TPCS2a After FUS Irradiation
Fluorescence microscopy was used to verify the uptake and intracellular localization of TPCS2a in the absence or presence of FUS irradiation (Figure 2). The sonosensitizer (red) is shown to be taken up in the F98 cells and localized in granular organelles representing endosomes and lysosomes (Figure 2a), as previously observed for other cell types. One-hour post FUS exposure (Figure 2b), a diffuse fluorescence throughout the cytosol was observed, indicating FUS-induced endosomal escape.
Figure 2: FUS-induced endosomal escape of TPCS2a. a) Sonosensitizer (red) was taken up in the F98 cells and was localized in the granular organelles representing endosome/lysosomes. b) 1-hour post FUS exposure shows diffuse TPCS2a indicating an induced endosomal escape of sonosensitizer by FUS irradiation.
II Effects of BLM and SDT on F98 Cells
In order to determine the optimal parameters for evaluating the effects of SCI on the viability and ability to form colonies of F98 cells, titrations of drug concentration and FUS sonication parameters (energy and irradiance) were performed. The colony growth and cell viability data illustrated in (Figure 3) show that F98 cells are sensitive to increasing BLM concentrations: the LD50 for BLM is approximately 0.4 μg/ml.
Figure 3:Effects of BLM on colony formation and cell viability. Both viability assays were compared to a control with varying BLM concentrations (0-0.6 µg/ml). a) 100 cells were seeded, and viability was recorded at each BLM concentration. b) Cell viability was recorded for each BLM concentration using an MTS assay. Error bars denote standard error.
In addition to varying BLM concentrations, the cells were also exposed to SDT with varying FUS irradiance and energy. As shown in (Figure 4), the highest FUS irradiances (0.3 and 0.4 W/cm2) resulted in significant cytotoxicity. Almost all cells exposed to SDT at 0.4 W/cm2 for 2 min. (48 J/cm2) were killed. Based on the data presented in (Figure 4), exposure of TPCS2a-incubated F98 cells to 2 min of 0.1 and 0.2 W/cm2 FUS resulted in cell survival/viability ranging between 35 and 80%, and as such, these exposure conditions were deemed appropriate for the SCI studies.
III SCI Mediated BLM Effects on F98 Cells
Two SCI methods were compared to BLM controls, as shown in (Figure 5). The SCI experiments were performed with a FUS irradiance of either 0.1 W/cm2 for 2 min or 0.2 W/cm2 for 1 min. The results show that SCI is significantly more effective compared to the BLM controls. As expected, SCI efficacy is sensitively dependent on BLM concentration: cell survival/viability decreases with increasing drug dose. Interestingly, at the highest drug dose (0.3 µg/ml), no statistically significant difference in efficacy was observed between the two SCI methods (0.2 W/cm2; 1 min vs. 0.1 W/cm2; 2 min) suggesting that efficacy at higher drug doses is dependent on the total energy delivered (12 J/cm2 in both cases).
Figure 4: Effects of SDT on F98 cells as a function of FUS irradiation parameters. SDT effect compared to a non-treated control with varying FUS exposure (0.1-0.4 W/cm2) over an interval of 1 or 2 min using: a) colony assay, or b) MTS assay. Asterisks (*) denote significant differences (p<0.05). Each data point represents the mean of four experiments. Error bars denote standard errors.
Figure 5: Effects of BLM and SCI on colony formation and cell viability. SCI effect compared to a BLM control with varying FUS exposure (0-0.2 W/cm2) over an interval of 1 or 2 min. Both viability assays were compared to a control with increasing BLM concentration from 0.075-0.3 µg/ml. a) Colony assay. b) MTS assay. Asterisks (*) denote significant differences (p<0.05). Each data point represents the mean of four experiments. Error bars denote standard errors.
Even in the absence of sonosensitizer, FUS exposure increased the efficacy of BLM (BLM vs. BLM+FUS group), as seen in (Figure 6) for both colony and cell viability assays. Since SCI is a technique that relies on the combination of drug, sonosensitizer and FUS exposure, the resultant toxicities should show more than an additive effect of the single modalities.
Figure 6: Effects of BLM, BLM+FUS and BLM - SCI on cell colony and cell viability. Both assays were compared to a control with varying BLM concentration from 0-0.3 µg/ml. (a) Colony assay. (b) MTS assay. Asterisks (*) denote significant differences (p<0.05). Each data point represents the mean of four experiments. Error bars denote standard errors.
The degree of synergism was calculated by comparing the survival fractions of BLM or SDT alone with that obtained for FUS+BLM or BLM-SCI using the cell survival data from (Figure 6b) at a sonication level of 0.2 W/cm2 - 1 min. As evidenced from the calculated αvalues, SCI demonstrated a pronounced synergistic effect of α = 3.3 and 3.9 for 0.15 and 0.3 µg/ml BLM respectively (the higher the α value, the greater the degree of synergism). Calculatedα values in the case of FUS+BLM were 1.4 and 1.36 for 0.15 and 0.3 µg/ml respectively. These results clearly demonstrated an increased synergistic effect of SCI compared to SDT or FUS+BLM applied independently.
Discussion
The limited penetration depth of light in biological tissues poses a significant challenge to light-based therapies such as PDT and PCI. In contrast, SDT and SCI have the potential to treat lesions deep within tissues and through the intact human skull. SDT has been extensively studied over a period of three decades in both in vitro and in vivo experiments. [12-17]. On the other hand, SCI is a relatively new therapeutic modality: the first studies were published in 201 [18]. Madsen et al. and Gonzalez et al. employed the combination of FUS+AlPcS2a+BLM in in vitro glioma models consisting of either glioma monolayers or 3-D multicellular spheroids, while Osaki et al. investigated the efficacy of SCI in vitro using a murine colon cell line and in vivo employing a murine mammary tumor model [6, 7, 19, 20].
The primary aim of the present study was to investigate the ability of SCI to increase the efficacy of BLM in a rat glioma cell line using the sono-photosensitizer TPCS2a. TPCS2a was developed in order to overcome some of the limitations associated with AlPcS2a, which has traditionally been used in PCI studies. Due to its strong absorption in the far-red region of the electromagnetic spectrum, AlPcS2a is especially useful for preclinical studies; however, it contains a large number of isomers with resulting batch-to-batch variations which may result in differences in clinical response [5, 9, 10]. TPCS2a was developed to address key clinical drug requirements including purity and reproducibility. Furthermore, the photobleaching rate of TPCS2a has been found to be much greater than AlPcS2a, which may be beneficial in degrading photosensitizer remaining in the skin. TPCS2a-induced PCI of BLM was recently evaluated in a phase I clinical trial in patients with local, recurrent, advanced, or metastatic cutaneous or subcutaneous malignancies [21]. The treatment was found to be safe and tolerable by all patients, and a TPCS2a dose of 0.25 mg/kg was recommended for future trials.
Results shown in (Figure 2) suggest that FUS irradiation induces endosomal escape of TPCS2a and is consistent with observations of Madsen et al. (6) and Gonzalez et al. (7) who obtained similar results for AlPcS2a in F98 monolayers. The results shown in (Figure 6) suggest that FUS potentiates the cytotoxic effects of BLM in both the absence and presence of TPCS2a compared to drug alone. The increased efficacy of FUS in the absence of the sonosensitizer is likely due to the sonoporation of the cell membrane [22-25]. In the presence of TPCS2a, the FUS-mediated-enhanced inhibitory growth effect of BLM is likely due to endosomal escape, in a similar manner to PCI.
Due to the different sonosensitizer concentrations used (0.3 µg/ml vs. 1 µg/ml for AlPcS2a) and the different FUS irradiation parameters, a direct efficacy comparison between the two sonosensitizers is somewhat difficult. Even so, a reasonable estimate may be obtained by comparing alpha values, which represent the degree of synergism between multiple modalities. Based on such a comparison, the AlPcS2a - SCI results of Gonzales et al. in an F98 spheroid model (7) were found to be in qualitative agreement with the TPCS2a - SCI findings of this study. For example, Gonzalez et al. reported an alpha value of 3.3 (0.5 µg/ml BLM; 0.2 W/cm2; 36 J/cm2) which compares favorably with the value (3.9) calculated in this study under somewhat similar conditions (0.3 µg/ml BLM; 0.2 Wcm2; 12 J/cm2).
It is thought that the mechanism underlying both cell plasma and endosome disruption is similar for both PCI and SCI, which are based on PDT and SDT effects, respectively. It should be noted that there is still some uncertainty as to the exact mechanism of the effects of SDT on cells, although ultrasound-induced inertial cavitation is likely a key component in the production of singlet oxygen via sonoluminescence emission [17, 18, 26, 27]. According to this hypothesis, indirect photoactivation of the sensitizing drug results in singlet oxygen generation. TPCS2a has a significant absorption peak around 400 nm, which overlaps with the most intense region of the sonoluminescence emission spectrum (α 200 to 400 nm), thus providing the possibility of significant singlet oxygen formation.
Conclusion
Fimaporfin mediated SCI of BLM significantly increased its efficacy several folds. The values obtained showed a significant degree of synergism of BLM-SCI compared to SDT, FUS activation of BLM or BLM alone. The noninvasive nature of FUS has the potential to lower drug concentrations and the possibility of repetitive fractionated treatment lowering the toxic side effects of chemotherapy. Unlike light-based treatment modalities, SCI is not limited by the poor tissue penetration inherent to light transmission.
Funding
The authors are grateful for support from the Norwegian Radium Hospital Research Foundation. Grant nr. SE.1503.
Steen Madsen acknowledges the support of the Tony and Renee Marlon Charitable Foundation.
Conflicts of Interest
None.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Abbreviations
FUS: Focused ultrasound
SDT: sonodynamic therapy
SCI: sonochemical internalization
PDT: photodynamic therapy
PCI: photochemical internalization
TPCS2a: fimaporfin
BLM: bleomycin
AlPcS2a: aluminium phthalocyanine disulfonate
TPPS2a: meso-tetraphenyl porphyrin disulfonate
Article Info
Article Type
Research ArticlePublication history
Received: Tue 05, May 2020Accepted: Mon 18, May 2020
Published: Fri 29, May 2020
Copyright
© 2023 Jimmy Nguyen Le. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.05.10
Author Info
Anders Høgset Henry Hirschberg Jimmy Nguyen Le Kristian Berg Odrun A Gederaas Steen J Madsen
Corresponding Author
Jimmy Nguyen LeBeckman Laser Institute and Medical Clinic, University of California, Irvine, California, USA
Figures & Tables






References
- Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K et al. (1999) Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res 59: 1180-1183. [Crossref]
- Norum OJ, Giercksky KE, Berg K (2009) Photochemical internalization as an adjunct to marginal surgery in a human sarcoma model. Photochem Photobiol Sci 8: 758-762. [Crossref]
- Selbo PK, Weyergang A, Høgset A, Norum OJ, Berstad MB et al. (2010) Photochemical internalization provides time-and space-controlled endolysosomal escape of therapeutic molecules. J Control Release 148: 2-12. [Crossref]
- Mathews MS, Blickenstaff JW, Shih EC, Zamora G, Vo V et al. (2012) Photochemical internalization of bleomycin for glioma treatment. J Biomed Opt 17: 058001. [Crossref]
- Jerjes W, Theodossiou TA, Hirschberg H, Høgset A, Weyergang A et al. (2020) Photochemical Internalization for Intracellular Drug Delivery. From Basic Mechanisms to Clinical Research. J Clin Med 9: E528. [Crossref]
- Madsen SJ, Gonzales J, Zamora G, Berg K, Nair RK et al. (2016) Comparing the Effects of Light- or Sonic-Activated Drug Delivery: Photochemical/ Sonochemical Internalization. J Environ Pathol Toxicol Oncol 35: 91-98. [Crossref]
- Gonzales J, Nair RK, Madsen SJ, Krasieva T, Hirschberg H (2016) Focused ultrasound-mediated sonochemical internalization: an alternative to light-based therapies. J Biomed Opt 21: 078002. [Crossref]
- Hirschberg H, Madsen SJ (2017) Synergistic efficacy of ultrasound, sonosensitizers and chemotherapy: a review. Ther Deliv 8: 331-342. [Crossref]
- Berg K, Nordstrand S, Selbo PK, Tran DT, Angell Petersen E et al. (2011) Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochem Photobiol Sci 10: 1637-1651. [Crossref]
- Wang, JT, Berg K, Hogset A, Bown SG, MacRobert AJ (2013) Photophysical and photobiological properties of a sulfonated chlorin photosensitiser TPCS(2a) for photochemical internalisation (PCI). Photochem Photobiol Sci 12: 519-526. [Crossref]
- Gederaas OA, Hauge A, Ellingsen PG, Berg K, Altin D et al. (2015) Photochemical internalization of bleomycin and temozolomide – in vitro studies on the glioma cell line F98. Photochem Photobiol Sci 14: 1357-1366. [Crossref]
- Umemura S, Yumita N, Nishigaki R, Umemura K (1990) Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res 81: 962-966. [Crossref]
- Nonaka M, Yamamoto M, Yoshino S, Umemura S, Sasaki K et al. (2009) Sonodynamic therapy consisting of focused ultrasound and photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res 29: 943-950. [Crossref]
- Jeong EJ, Seo SJ, Ahn YJ, Choi KH, Kim KH et al. (2012) Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med Biol 38: 2143-2150. [Crossref]
- Costley D, Mc Ewan C, Fowley C, Mchale AP, Atchison J et al. (2015) Treating cancer with sonodynamic therapy: A review. Int J Hyperthermia 31: 107-117. [Crossref]
- McHale AP, Callan JF, Nomikou N, Fowley C, Callan B (2016) Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. Adv Exp Med Biol 880: 429-450. [Crossref]
- Choi V, Rajora MA, Zheng G (2020) Activating Drugs with Sound: Mechanisms Behind Sonodynamic Therapy and the Role of Nanomedicine. Bioconjug Chem 31: 967-989. [Crossref]
- Hirschberg H, Gonzales J, Nair RK, Madsen SJ (2015) Ultrasonic irradiation to enhance chemotherapy. SPIE Newsroom.
- Osaki T, Ono M, Uto Y, Ishizuka M, Tanaka T et al. (2016) Sonodynamic therapy using 5-aminolevulinic acid enhances the efficacy of bleomycin. Ultrasonics 67: 76-84. [Crossref]
- Osaki T, Yokoe I, Uto Y, Ishizuka M, Tanaka T et al. (2016) Bleomycin enhances the efficacy of sonodynamic therapy using aluminum phthalocyanine disulfonate. Ultrason Sonochem 28: 161-168. [Crossref]
- Sultan AA, Jerjes W, Berg K, Høgset A, Mosse CA et al. (2016) Disulfonated tetraphenyl chlorin (TPCS2a)-induced photochemical internalisation of bleomycin in patients with solid malignancies: a phase 1, dose-escalation, first-in-man trial. Lancet Oncol 17: 1217-1229. [Crossref]
- Pepe J, Rincon M, Wu J (2004) Experimental comparison of sonoporation and electroporation in cell transfection applications. Acoust Res Lett Onl 5: 62-67.
- Yudina A, Lepetit Coiffé M, Moonen CT (2011) Evaluation of the temporal window following ultrasound-mediated cell membrane permeability enhancement. Mol Imaging Biol 13: 239-249. [Crossref]
- Wu J, Pepe J, Rincon M (2006) Sonoporation, anti-cancer drug and antibody delivery using ultrasound. Ultrasonics 44: e21-e25. [Crossref]
- Kessel D, Jeffers R, Fowlkes JB, Cain C (1994) Porphyrin-induced enhancement of ultrasound toxicity. Int J Rad Biol 66: 221-228. [Crossref]
- Putterman SJ, Weninger KR (2000) Sonoluminescence: How bubbles turn sound into light. Annu Ref Fluid Mech 32: 445-476.
