Two-Way Relationship Between Helicobacter Pylori Infection and Periodontitis: Results from A Systematic Review and Meta-Analysis
A B S T R A C T
Aim: Helicobacter pylori (H. pylori) infection and periodontitis have considerable worldwide prevalence once they both present systemic alterations with a possible association between them. Therefore, we have performed this meta-analysis to assess the possible association between H. pylori infection and periodontitis.
Material and Methods: A systematic search in the literature was performed for studies published before December 2, 2019 in diverse scientific and educational databases. The data was extracted by two investigators and the statistical analysis was performed by Review Manager statistical program with heterogeneity and Odds Ratio (OR) with 95% of Confidence Intervals (CI) calculations as well as a sensitive analysis to assess the accuracy of the results. The value of P<0.05 was considered as significant. In addition, we performed the analysis of the quality of included studies as well as the evaluation for risk of bias.
Results: In overall analysis, H. pylori infection was associated with the risk of periodontitis development (OR = 1.72, CI: 1.47, 2.02, P<0.00001) and the periodontitis was considered as a risk factor for H. pylori infection (OR = 3.21, CI: 2.31, 4.47, P<0.00001). Moreover, the evaluation of dental plaque from patients with periodontitis reveled increased risk of H. pylori infection (OR = 3.46, CI: 2.39, 5.01, P<0.00001).
Conclusions: This current systematic review and meta-analysis composed by 12 studies in 7,059 participants showed that H. pylori infection increased significantly the risk of the development of periodontitis and the periodontitis may be a risk for this bacterial infection.
Keywords
Periodontal disease, gastrointestinal tract, odds ratio, periodontal medicine, risk factor
Introduction
Helicobacter pylori (H. pylori) are gram-negative, spiral-shaped and microaerophilic microorganisms that colonize the human gastric-intestinal tract, specifically the stomach [1]. This bacteria species is commonly transmitted by oral-fecal way within family since early childhood with possibly persistence for decades in the gastric mucosa despite the host immune response [2]. This microorganism is already associated with several gastric disorders from gastritis to gastric cancer [3, 4]. In the medical clinic scenario, several types of diagnostic methods are available to detect H. pylori infection, such as: histological evaluation, Rapid Urease Test (RUT), molecular technics, serological kits and culture [5-7].
H. pylori infection also was associated with several others extra gastric conditions [8]. Studies available in literature report the relationship among H. pylori infection and heart failure hepatic insulin resistance pregnancy-related disorders and oral diseases [9-12]. Results from a meta-analysis showed a significant association between halitosis and H. pylori present in stomach with an Odds Ratio value of 4.03 [13].
In the field of oral diseases, there is the periodontitis, characterized as an immune-inflammatory disease as response to the presence of microorganisms in periodontal sites [14]. The pathogenesis of periodontitis receives influence of several factors including the colonization of periodontal sites by gram-positive and gram-negative bacteria species oral hygiene and genetic variations [15-19]. Both periodontitis and H. pylori infection had considerable worldwide prevalence and are associated with systemic conditions [8, 20-23].
In a recent case-control study, the possible association between H. pylori infection and risk of periodontitis was demonstrated which the H. pylori infection has represented an exposure influencing the outcome of periodontitis [24]. Indeed, others previous studies also have evaluated this association as well as have demonstrated the considerable prevalence of this microorganism in oral cavity [25-27]. However, other authors showed the inverse relationship, which the periodontal inflammation with the presence of H. pylori infection in periodontal pockets is indicated as source of the microorganism maintenance in human body and in the recurrence of gastric disorders even after an antibiotic therapy [25].
Up to the present moment, there has not been a study that gathered the results about H. pylori infection and periodontitis with an increased power of statistical association and which have demonstrated the real relationship between these two conditions. Given the lack of this information, this study aimed at performing a meta-analysis to assess the possible association between H. pylori infection and the risk of periodontitis based on case-control studies, observational studies or cross-sectional studies.
Material and Methods
This systematic review and meta-analysis is registered in PROSPERO database with the following ID number: CRD42017073192.
I Clinical Question Research
What are the real risk and association between Helicobacter pylori infection and periodontitis? May Helicobacter pylori infection be a risk for periodontitis or is periodontitis correlated with Helicobacter pylori infection?
II PICO Statement
The PICO statement used was the following [28]:
P – Patients, problem or population: Positive patients for H. pylori infection, patients diagnosed with periodontitis and healthy periodontal controls.
I – Intervention: Collection of the frequency of H. pylori infection between patients with periodontitis and control groups.
C – Comparison: The influence between the presence of H. pylori and periodontitis
O – Outcome: Odds Ratio calculation to measure the association between H. pylori infection and periodontitis and to assess the periodontitis as risk for H. pylori infection.
III Protocols Used
This meta-analysis followed the recommended PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [29]. The screened studies were evaluated by Newcastle-Ottawa scale for quality assessment studies with score < 7 were excluded [30]. This study (systematic review and meta-analysis) does not require approval by ethics committee.
IV Eligibility Criteria
Articles were included in the current meta-analysis if the studies met all the following criteria: 1) Evaluation of Helicobacter pylori infection with occurrence of periodontitis in humans; 2) Case patients received diagnosis of chronic, aggressive periodontitis or localized aggressive periodontitis and control patients had healthy periodontal evaluation; 3) Diagnosis of the periodontitis confirmed through clinical manifestations or radiographic findings as previously described and diagnosis of Helicobacter pylori infection confirmed by previous accurate methods and; 4) participants enrolled: analysis did not present pregnancy or previous systemic disorders such as diabetes or auto-immunity disease [5, 6, 31].
V Search Strategy
A comprehensive search in literature was performed by two investigators for studies that approached the association between H. pylori infection and periodontal disease. Google Scholar, MedLine, PubMed and Web of Science were the medical and scientific databases used in the literature retrieval. The following combined keywords or Medical Subject Headings (MeSH) were used: [(Helicobater pylori or H. pylori)] and [(infection, gastric disorder]) and (periodontitis or periodontal disease or chronic periodontitis or aggressive periodontitis)]. There was no language restriction in the search strategy that approached studies published before December 2, 2019. The abstracts from included studies, as well as their references, were screened by investigators to identify potential additional studies.
VI Data Collection Process
Two investigators (FRPS and JGG) independently reviewed all studies and extracted the data using a standardized form. The data were collected on the first author, year of publication, study design, sample size, country, source of sample, method of detection of H. pylori infection and obtained score to assess the quality of included studies.
VII Statistical Analysis
The statistical analysis of the data was performed with the Review Manager software version 5.3 (RevMan, Nordic Cochrane Centre, The Cochrane Collaboration, 2012). The chi-squared Q-based statistical test (I²) was used to assess the presence of heterogeneity with evaluation of the Funnel plot for heterogeneity. When the value of I² was not statistically significant (I²<50%, P>0.05) the Fixed-effect model was used to estimate the pooled Odds Ratio (OR). On the other hand, when heterogeneity was significant (I²>50%, P<0.05) the Random-effects model was used for the OR calculation. In both methods, the P value <.05 was considered statistically significant. In addition, a sensitivity analysis was also performed to test the robustness of the results pooled by omitting one included study at a time to detect individual effects on the overall analyses and the risk of bias were assessed by use of the Cochrane Collaboration’s tool [32]. All the data in the studies were dichotomous data expressed as OR with 95% of confidence intervals (CI) to assess the association between H. pylori infection and periodontitis.
Table 1: Characteristics of included studies in this systematic review and meta-analysis.
|
First author |
Year |
Sample size (Cc/Co) |
Country |
Source of sample |
Method of detection |
Subject type |
Score |
|
|
Al Asquah |
2009 |
HP+ |
HP- |
Saudi Arabia |
Hospital |
RUT |
Dental plaque - Stomach |
8 |
|
Anand |
2006 |
37/13 |
25/26 |
India |
Community |
RUT and Serological test |
- |
9 |
|
Ding |
2015 |
541/73 |
312/62 |
China |
Hospital |
HPS |
- |
8 |
|
Dye |
2002 |
30/35 |
20/49 |
United States |
Community |
Serological test |
Whites – Blacks – Mexican |
9 |
|
Navabi |
2010 |
189/951 |
463/3,981 |
Iran |
Hospital |
RUT |
Men - Women |
8 |
|
Nisha |
2016 |
23/17 |
12/13 |
India |
Community |
RUT |
- |
8 |
|
Silva |
2010 |
209/136 |
84/71 |
Brazil |
Hospital |
PCR |
- |
7 |
|
Souto |
2008 |
12/1 |
50/30 |
Brazil |
Community |
PCR |
- |
9 |
|
Umeda |
2003 |
39/4 |
130/52 |
Japan |
Hospital |
PCR |
- |
8 |
|
Yang |
2016 |
7/1 |
10/10 |
China |
Hospital |
RUT, culture and PCR |
- |
8 |
|
Zheng and Zhou (1) |
2015 |
50/20 |
24/46 |
China |
Hospital |
PCR |
- |
8 |
|
Zheng and Zhou (2) |
2015 |
25/45 |
9/61 |
China |
Hospital |
PCR |
- |
8 |
Cc – Case patients for periodontitis, Co – Control patients, HP+ – Helicobacter pylori-positive patients, HP- – Helicobater pylori-negative patients, RUT- Rapid Urease Test kit, HPS – saliva Helicobacter pylori antigen test, PCR – Polimerase Chain Reaction
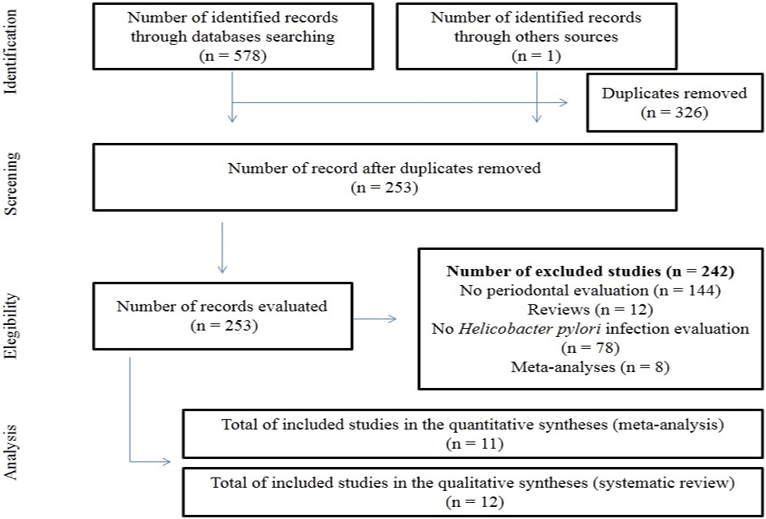
Figure 1: Flowchart for identification, screening, eligibility and analysis of included studies in this current meta-analysis.
Results
I Characteristics of Studies Included in the Systematic Review
The PRISMA checklist for this current systematic review and meta-analysis is available as supplementary material (Table S1). At the end of the systematic search, 11 articles [12, 25-26, 33-39] met the inclusion criteria and, therefore, composed the meta-analysis (Figure 1). One article stratified the results in two different PCR methods for H [39]. pylori diagnosis. Seen this data, we considered that this article was composed by two studies. Therefore, this current systematic review brings 11 articles with 12 studies.
The studies were published between 2003 and 2016 and enrolled 7,059 participants. Diverse ethnical groups composed the studies and the patients were enrolled both in a Hospital-based population and a Community-based population [12, 24-26, 33-39]. The studies used four types of diagnosis methods for H. pylori detection: Rapid Urease test (RUT) Serological test to antibody against the bacteria Polymerase Chain Reaction (PCR) and saliva H. pylori antigen test (HPS) [12, 24, 25, 33-35, 37-39]. Tree studies focused in to assess the H. pylori infection as potential risk factor for periodontitis [12, 24, 33]. On the other hand, eight studies were conducted to clarify the periodontitis as risk factor for H. pylori infection [25, 26, 34-39]. All studies presented more than seven points in the score scale and were included in the results. The Table 1 shows the main characteristics of the included studies.
II Meta-Analysis
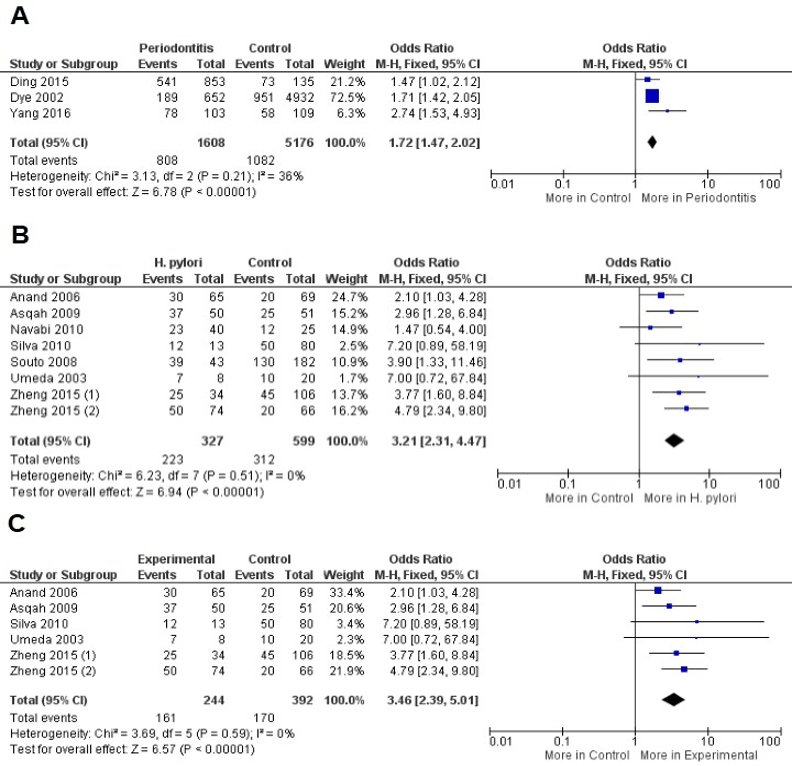
The presence of H. pylori was associated with the risk of periodontitis development in overall analysis (OR = 1.72, CI: 1.47, 2.02, P<0.00001) with non-significant value of heterogeneity (I2 = 36%, P = 0.21). Similar results were observed when periodontitis was evaluated as risk factor for H. pylori infection (OR = 3.21, CI: 2.31, 4.47, P<0.00001) also with a non-significant value of heterogeneity (I2 = 0%, P = 0.51). The evaluation of dental plaque from patients with periodontitis reveled increased risk of H. pylori infection (OR = 3.46, CI: 2.39, 5.01, P<0.00001) with decreased value of heterogeneity (I2 = 0%, P = 0.59). Due to the non-significant value of heterogeneity in the included calculations, the Fixed-effect statistical model was applied (Figure 2).
Figure 2: A) Forest plot to comparison between patients with periodontitis and control to H. pylori infection in overall analysis, B) Periodontitis as risk factor for H. pylori infection and C) Dental plaque from patients with periodontitis and H. pylori infection.
III Sensitive Analysis and Risk of Bias
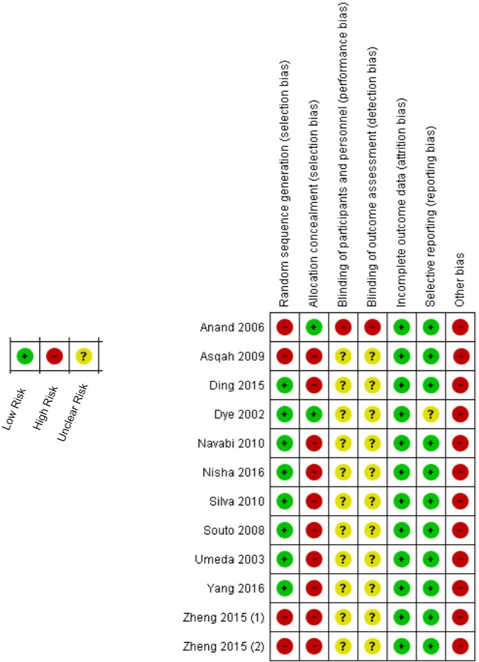
The sensitive analysis showed that the study performed by Nisha et al. changed significantly the results in which considerable increase in heterogeneity was observed (I² = 55%, Pheterogeneity = 0.02) [38]. When this study was excluded the heterogeneity has decreased to be unremarkable (I² = 0%, Pheterogeneity = 0.51). Therefore, 11 studies have participated of the meta-analysis calculations. The risk of bias summary based in the Cochrane Collaboration’s tool was showed in (Figure 3), in which all included studies failed to approach the several types of periodontitis classifications and, so, they presented elevated risk of others bias.
Discussion
To the best of our knowledge, this is the first meta-analysis to focus on the association between H. pylori and periodontitis as a two-way relationship. Some meta-analyses focused on determining the association between H. pylori infection and extra gastric disorders such as: diabetes or halitosis [13, 40]. However, a specific two-way association between H. pylori infection and periodontitis by a complete meta-analysis has never been in the literature so far. Our meta-analysis showed that H. pylori might increase the risk of periodontitis development (Figure 2). H. pylori are a known microorganism that cause gastro-intestinal disturbances [3, 4]. The studies showed the capacity of H. pylori to promote stimulation to release interleukin-8 and a specific group of proteins associated with the periodontitis progression [41]. Although some authors suggested that this bacterial species might be in a commensal relation with the human body when present in the oral cavity, other subsequent study showed that H. pylori has an important role in oral disturbances [42, 43]. H. pylori may induce periodontal inflammation by the creation of antigen-antibody complexes who infiltrate in the gingival sulcus with periodontal inflammation [44].
On the other hand, the own periodontitis may be a risk factor for H. pylori infection (OR = 3.21, P<0.00001). Results from a previous meta-analysis demonstrated that the periodontal therapy appears to reduce the gastric H. pylori recurrence [45]. Besides, other data available in the literature demonstrated that the dental plaque control was effective in preventing H. pylori to induce gastric disease [45, 46]. Despite these findings, H. pylori were detected more in supragiginval plaque rather than in subgingival plaque, what may be signal that this bacterium species does not commonly colonize the periodontal pockets [37]. These authors are according with our results that demonstrated the increased risk of H. pylori infection in dental plaque from patients with periodontitis (Figure 2). The dental plaque is a biofilm that adheres to teeth as well as others surfaces in the oral cavity promoting an interesting advantage composed by the resistance to host immune response; Therefore, H. pylori as biofilm-associated is protected from host immune response and systemic antibiotics therapy [47].
Taken in base the evaluation by ethnicity, six studies performed the evaluation of H. pylori infection and periodontitis in Asian ethnicity, more specifically in India China and Japan and two studies were carried out among Brazilians [12, 24, 25, 34, 35, 37-39]. Anand et al. evaluated 134 subjects to assess the relationship between oral conditions and H. pylori infection. They detected 65 patients positive for H. pylori infection and 46.2% of these patients had periodontitis. H. pylori infection already had a significant prevalence in this population [48]. The high prevalence of H. pylori infection, as well as periodontitis, in populations from Asian and South America leads us to pay more attention in our results seen the limited number of included studies that have been performed in these aforementioned populations [20, 49].
This is the first meta-analysis focused on assessing the association between H. pylori infection and periodontitis, in which the results may be considered accurate by non-significant heterogeneity in the performed analyses. However, the meta-analysis has presented some important limitations that should be noted. First, 11 studies composed the results. Although a considerable number of patients was enrolled (Table 1), more studies are required to better evaluating the association between this bacterial infection and periodontitis. Second, H. pylori not only are present in patients that manifest gastric symptoms, but also in asymptomatic infected health patients [50]. Hence, the patients with periodontitis may be not diagnosed to H. pylori infection and therefore are not included in the studies. Third, periodontitis itself has notable variations in clinical manifestations, diagnosis and therapeutic approaches; insufficient data brought by included studies did not enable a better evaluation due to such characteristics and may represent a potential bias as showed in (Figure 3). Fourth, two relationships have been approached in the included studies: whether the presence of H. pylori may influence in periodontitis, taken this disease as an outcome or whether periodontitis may be a cause of infection or reinfection by H. pylori.
Figure 3: Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
We tried to considerate this question. However, future studies should be focused on assessing the prevalence of H. pylori infection among patients with periodontitis and on the health periodontal evaluation assessing whether both groups present gastric alterations. Moreover, further researches should perform the evaluation of H. pylori infection as an exposure to periodontitis and the inverse evaluation. Fifth, periodontitis is a heterogeneous inflammatory condition that receives as environmental influence as host genetic conditions [15-19, 51, 52]. In conclusion, this current systematic review and meta-analysis composed by 12 studies in 7,059 participants showed that H. pylori infection is associated with periodontitis and the disease may be considered as a risk factor for H. pylori infection.
Conflicts of Interest
The authors declare there is any conflict of interest in this research.
Article Info
Article Type
Review ArticlePublication history
Received: Thu 02, Jan 2020Accepted: Fri 17, Jan 2020
Published: Thu 23, Jan 2020
Copyright
© 2023 Felipe Rodolfo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CEI.2020.01.03
Author Info
Silvania Conceição Furtado Alessandro Luiz Araújo Bentes Leal Any Carolina Cardoso Guimarães Vasconcelos Daniel Fernando Pereira Vasconcelos Felipe Rodolfo José Fernando Marques Barcellos Juliana Gomes Galeno Larissa dos Santos Pessoa Reyce dos Santos Koga Zinalton Gomes de Andrade
Corresponding Author
Felipe RodolfoDoctorate Student Post-Graduation Program in Basic and Applied Immunology, Federal University of Amazonas, Manaus, Brazil
Figures & Tables
Table 1: Characteristics of included studies in this systematic review and meta-analysis.
|
First author |
Year |
Sample size (Cc/Co) |
Country |
Source of sample |
Method of detection |
Subject type |
Score |
|
|
Al Asquah |
2009 |
HP+ |
HP- |
Saudi Arabia |
Hospital |
RUT |
Dental plaque - Stomach |
8 |
|
Anand |
2006 |
37/13 |
25/26 |
India |
Community |
RUT and Serological test |
- |
9 |
|
Ding |
2015 |
541/73 |
312/62 |
China |
Hospital |
HPS |
- |
8 |
|
Dye |
2002 |
30/35 |
20/49 |
United States |
Community |
Serological test |
Whites – Blacks – Mexican |
9 |
|
Navabi |
2010 |
189/951 |
463/3,981 |
Iran |
Hospital |
RUT |
Men - Women |
8 |
|
Nisha |
2016 |
23/17 |
12/13 |
India |
Community |
RUT |
- |
8 |
|
Silva |
2010 |
209/136 |
84/71 |
Brazil |
Hospital |
PCR |
- |
7 |
|
Souto |
2008 |
12/1 |
50/30 |
Brazil |
Community |
PCR |
- |
9 |
|
Umeda |
2003 |
39/4 |
130/52 |
Japan |
Hospital |
PCR |
- |
8 |
|
Yang |
2016 |
7/1 |
10/10 |
China |
Hospital |
RUT, culture and PCR |
- |
8 |
|
Zheng and Zhou (1) |
2015 |
50/20 |
24/46 |
China |
Hospital |
PCR |
- |
8 |
|
Zheng and Zhou (2) |
2015 |
25/45 |
9/61 |
China |
Hospital |
PCR |
- |
8 |
Cc – Case patients for periodontitis, Co – Control patients, HP+ – Helicobacter pylori-positive patients, HP- – Helicobater pylori-negative patients, RUT- Rapid Urease Test kit, HPS – saliva Helicobacter pylori antigen test, PCR – Polimerase Chain Reaction
References
- Lopes AL, Vale FF, Oleastro M (2014) Helicobacter pylori infection – recent developments in diagnosis. World J Gastroenterol 20: 9299-9313. [Crossref]
- Salama NR, Hartung ML, Müller A (2013) Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol 11: 385-399. [Crossref]
- Watari J, Chen N, Amenta PS, Fukui H, Oshima T et al. (2014) Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 20: 5461-5473. [Crossref]
- Graham DY (2015) Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 148: 719-731. [Crossref]
- Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ (2014) A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol 20: 1438-1449. [Crossref]
- Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G (2014) Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol 20: 12847-12859.
- Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY et al. (2015) Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol 21: 11221-11235. [Crossref]
- Franceschi F, Gasbarrini A, Polyzos SA, Kountouras J. (2015) Extragastric diseases and Helicobacter pylori. Helicobacter 1: 40-46. [Crossref]
- Matsushima S, Tsuda M, Matsumoto J, et al. (2016) Association of Helicobacter Pylori with Chronic Heart Failure. J Card Fail 2: S181.
- Zhou X, Liu W, Gu M, et al. (2015) Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol 50: 1027-1040. [Crossref]
- Cardaropoli S, Rolfo A, Todros T (2014) Helicobacter pylori and pregnancy-related disorders. Word J Gastroenterol 20: 654-664. [Crossref]
- Ding YJ, Yan TL, Hu XL, Liu JH, Yu CH et al. (2015) Association of salivary Helicobacter pylori infection with oral diseases: a cross-sectional study in a Chinese population. Int J Med Sci 12: 742-747. [Crossref]
- Dou W, Li J, Xu L, Zhu J, Hu K et al. (2016) Halitosis and helicobacter pylori infection: A meta-analysis. Medicine (Baltimore) 95: e4223. [Crossref]
- Knight ET, Liu J, Seymour GJ, Faggion CM Jr, Cullinan MP (2016) Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontol 2000 71: 22-51. [Crossref]
- Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15: 30-44. [Crossref]
- Lertpimonchai A, Rattanasiri S, Arj-Ong Vallibhakara S, Attia J, Thakkinstian A (2017) The association between oral hygiene and periodontitis: a systematic review and meta‐analysis. Int Dent J 67: 332-343. [Crossref]
- Marchesan JT, Jiao Y, Moss K, Divaris K, Seaman W et al. (2017) Common polymorphisms in the IFI16 and AIM2 genes are associated with periodontal disease. J Periodontol 88: 663-672. [Crossref]
- Silva FRP, Vasconcelos ACCG, França LFC, et al. (2017) Relationship between -889 C/T polymorphism in interleukin-1A gene and risk of chronic periodontitis: evidence from a meta-analysis with new published findings. Med Oral Patol Oral Cir Bucal 22: e7-e14.
- Da Silva FRP, Pessoa LDS, Vasconcelos ACCG, Lima WA, Alves EHP et al. (2017) Polymorphisms in interleukins 17A and 17F genes and periodontitis: results from a meta-analysis. Mol Biol Rep 44: 443-453. [Crossref]
- Susin C, Haas AN, Albandar JM (2014) Epidemiology and demographics of aggressive periodontitis. Periodontol 2000 65: 27-45. [Crossref]
- Eusebi LH, Zagari RM, Bazzoli F (2014) Epidemiology of Helicobacter pylori infection. Helicobacter 19: 1-5.
- França LFC, Vasconcelos ACCG, Silva FRP, Alves EHP, Carvalho JS et al. (2017) Periodontitis changes renal structures by oxidative stress and lipid peroxidation. J Clin Periodontol 44: 568-576. [Crossref]
- Vasconcelos DF, Silva FR, Pinto ME, Santana LA, Souza IG et al. (2017) Decrease of pericytes is associated with liver disease caused by ligature-induced periodontitis in rats. J Periodontol 88: e49-e57. [Crossref]
- Yang J, Zhang Q, Chen M, Wu WZ, Wang R et al. (2016) Association between Helicobacter pylori infection and risk of periodontal diseases in Han Chinese: a case-control study. Med Sci Monit 22: 121-126. [Crossref]
- Anand OS, Nadakumar K, Shenoy KT (2006) Are dental plaque, poor oral hygiene, and periodontal disease associated with Helicobacter pylori infection? J Periodontol 77: 692-698. [Crossref]
- Al Asqah M, Al Hamoudi N, Anil S, Al Jebreen A, Al-Hamoudi WK (2009) Is the presence of Helicobacter pylori in the dental plaque of patients with chronic periodontitis a risk factor for gastric infection? Can J Gastroenterol 23: 177-179. [Crossref]
- Gebara EC, Pannuti C, Faria CM, Chehter L, Mayer MP et al. (2004) Prevalence of Helicobacter pylori detected by polymerase chain reaction in the oral cavity of periodontitis patients. Oral Microbiol Immunol 19: 277-280. [Crossref]
- University Library [Internet]. Chicago: The University of Illinois at Chicago’s Library.
- Moher D, Liberatti, A, Tetziaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [Crossref]
- Weels G, Shea B, O’Connell D et al. (2019) Ottawa Hospital Research Institute [Internet]. Ottawa.
- Page RC, Eke PI (2007) Case definitions for use in population-based surveillance of periodontitis. J Periodontol 78: 1387-1399. [Crossref]
- Higgins JPT, Altman DG (2008) Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley 187-241.
- Dye BA, Kruszon-Moran D, McQuillian G (2002) The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am J Public Health 92: 1809-1815. [Crossref]
- Umeda M, Kobayashi H, Takeuchi Y, Hayashi J, Morotome-Hayashi Y et al. (2003) High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol 74: 129-134. [Crossref]
- Souto R, Colombo AP (2008) Detection of Helicobacter pylori by polymerase chain reaction in the subgingival biofilm and saliva of non-dyspeptic periodontal patients. J Periodontol 79: 97-103. [Crossref]
- Navabi N, Darvismoghadam S, Torabi M (2010) Association between gastric Helicobacter pylori infection and periodontal disease. J Dent (Shiraz) 10: 45-49.
- Silva DG, Stevens RH, Macedo JM, et al. (2010) Presence of Helicobacter pylori in supragingival dental plaque of individuals with periodontal disease and upper gastric diseases. Arch Oral Biol 55: 896-901. [Crossref]
- Nisha KJ, Nandakumar K, Shenoy KT, Janam P (2016) Periodontal disease and Helicobacter pylori infection: a community‐based study using serology and rapid urease test. J Investig Clin Dent 7: 37-45. [Crossref]
- Zheng P, Zhou W (2015) Relation between periodontitis and helicobacter pylori infection. Int J Clin Exp Med 8: 16741.
- Zhou X, Zhang C, Wu J, Zhang G (2013) Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract 99: 200-208. [Crossref]
- Hu Z, Zhang Y, Li Z, Yu Y, Kang W et al. (2016) Effect of Helicobacter pylori infection on chronic periodontitis by the change of microecology and inflammation. Oncotarget 7: 66700-66712. [Crossref]
- Song Q, Haller B, Ulrich D, Wichelhaus A, Adler G et al. (2000) Quantitation of Helicobacter pylori in dental plaque samples by competitive polymerase chain reaction. J Clin Pathol 53: 218-222. [Crossref]
- Loster BW, Majewski SW, Czesnikiewicz-Guzik M, Bielanski W, Pierzchalski P et al. (2006) The relationship between the presence of Helicobacter pylori in the oral cavity and gastric in the stomach. J Physiol Pharmacol 57: 91-100. [Crossref]
- Okuda K, Kimizuka R, Katakura A, Nakagawa T, Ishihara K (2003) Ecological and immunopathological implications of oral bacteria in Helicobacter pylori-infected disease. J Periodontol 74: 123-128. [Crossref]
- Bouziane A, Ahid S, Abouqal R, Ennibi O (2012) Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: a systematic review and meta‐analysis. J Clin Periodontol 39: 1166-1173. [Crossref]
- Jia CL, Jiang GS, Li CH, Li CR (2009) Effect of dental plaque control on infection of Helicobacter pylori in gastric mucosa. J Periodontol 80: 1606-1609. [Crossref]
- Anand PS, Kamath KP, Anil S (2014) Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World J Gastroenterol 20: 5639-5653. [Crossref]
- Adlekha S, Chadha T, Krishnan P, Sumangala B (2013) Prevalence of Helicobacter pylori infection among patients undergoing upper gastrointestinal endoscopy in a medical college hospital in Kerala, India. Ann Med Health Sci Res 3: 559-563. [Crossref]
- Shah N, Mathur VP, Kant S, Gupta A, Kathuria V et al. (2017) Prevalence of dental caries and periodontal disease in a rural area of Faridabad District, Haryana, India. Indian J Dent Res 28: 242-247. [Crossref]
- Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P (2014) Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomized controlled trials. BMJ 348: g3174.
- Silva MK, Carvalho ACG, Alves EHP, Silva FRP, Pessoa LS et al. (2017) Genetic Factors and the risk of periodontitis development: findings from a systematic review composed of 13 studies of meta-analysis with 71,531 participants. Int J Dent 2017: 1914073. [Crossref]
- Silva FRP, Vasconcelos ACCG, França LFC, Lenardo D, Nascimento HMS et al. (2018) Association between the rs1143634 polymorphism in interleukin-1B and chronic periodontitis: results from a meta-analysis composed by 54 case/control studies. Gene 668: 97-106. [Crossref]
