Journals
Transplantation of enteric neural stem cells into rat digestive tract: potential therapy for aganglionosis
A B S T R A C T
Aim: Recently, lots of progress in the diagnosis and therapy of hirschsprung’s disease(HD), but curative treatment options are still limited, and the long-term outcome often remains unsatisfactory. The study of enteric neural stem cells(ENSCs) over the past decade opens up the possibility of definitive curative ther-apies for HD. The study was aimed to explore the possibility of survival of ENSCs transplantation into the gastrointestinal of rats.
Materials and methods: The study included 3-4 weeks old male SD rats and the ENSCs were obtained from the alimentary tract of the rats on day 20 of the embryonic stage. These were cultured in vitro, proliferated and labeled with PKH-26 and were transplanted into the gastrointestinal wall of rats by injection. Survival, morphology, and migration of ENSCs were observed by dynamic observation after transplantation.
Results and Conclusions: Morphology and immunology analysis showed cell colony formation, NLBs appeared. Labeled ENSCs by PKH-26 showed normal morphology in the gastrointestinal walls. The distribution of the grafted cells was irregular primarily, became regular, and migrated towards the submucosa. Some grafted cells expressed TUJ-1, considered to be one of the earliest signs of stem cell differentiation into neurons. Grafted cells survived well for at least 2 weeks. Transplanted ENSCs successfully engrafted into recipient digestive tract showing appropriate spread, differentiation and localisation. This study provides a potential therapy for aganglionosis.
K E Y W O R D S
Enteric neural stem cells, Transplantation, hirschsprung’s disease
Abbreviations
BSA, bovine serum albumin
ENSCs, enteric neural stem cells; ENS, enteric nervous system
GI, gastrointestinal
GFAP, glial fibrillary acidic protein; HD, Hirschsprung’s disease
HFN, human plasma fibronectin purified protein;
H&E, hematoxylin-eosin staining
NLB, Neurosphere-like bodies
PBS, phosphate-buffered saline
RT, room temperature; SD, Sprague-Dawley; SMA, smooth muscle actin
TUJ-1, tublin beta-III
I N T R O D U C T I O N
The ENS or the intrinsic nervous system of the GI tract, is responsible for the regulation of homeostatic key features in the GI tract, such as gastric motility, secretion and absorption [1, 2]. The ENS consists of an extensive ganglionic network of neurons and glial cells within the gut wall [3]. Approximately 400-600 million enteric neurons are present in humans and more than 1.2 million are found in mice [1, 4]. However, this complexity exposes the ENS to an array of congenital disorders that disrupt its development, and one of the most challenging GI disorders is the HD.
HD results from a failure of the complex multigenic interactions that underlie the development of the ENS from precursor cells derived from the neural crest during embryogenesis [5, 6]. This failure results in the incomplete rostro caudal colonization of the developing gut primarily by the cells derived from the vagal neural crest, and, in turn, resulting in the complete failure of the ENS formation (i.e., aganglionosis) in a segment of the distal intestine (the length of which varies among patients) [7]. The aganglionic bowel shows evidence of gut dysmotility, gut transit disorders etc. The condition maintains a state of tonic contraction for several minutes to hours presumably as a result from the absence of the inhibitory influences that are usually provided by the ENS, as well as from hypertrophied extrinsic nerve fibres, which are excitatory in animal models.
Although there has been much progress in the diagnosis and therapy of HD, curative treatment options are still limited, and the long-term outcome often remains unsatisfactory [8]. The study of neural stem cells over the past decade opens up the possibility of definitive curative ther-apies for HD. A number of recent studies have suggested that ENSCs have the potential to replace/restore missing or defective ENS cells in the aganglionic gut [8-12]. But it is still not clear whether these transplanted ENSCs can survive longer and migrate in the digestive tract.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s Medium-low glucose, N2-supplement, B27-supplement, Penn/Strep (Gibco Company, USA), PKH-26, Hoechst 33258, basic fibroblast growth factor(b-FGF), epidermal growth factor(EGF), collagenase, retinoic acid, (Sigma-Aldrich, USA), Chick Embryo Extract(CEE) (Accurate chemical, USA), 2-mereaptoethanol, HFN(Chemicon,USA), Rat-anti-TUJ-1 (R&D, USA), Rabitt-anti-Nestin, Rabitt-anti-GFAP and FITC, Cy-3 (Boster Company, China), Fluorescein-Conjugated affinipure goat anti-mouse IgG (H+L) (Beijing Zhongshan golden bridge biotechnology company, China).
Experimental animals
The experimental animal in vivo protocols were approved by the Ethics Committee of Wenzhou Medical University and were in line with the International guidelines for animal welfare. ENSCs were isolated from embryo (embryonic day 20). 3-4 weeks old (weight: 100-120g) male Sprague Dawley rats were used as recipient into which ENSCs were transplanted. They were all purchased from the Animal Center of Wenzhou Medical University. 20 rats were divided into 2 groups in random, pylorus transplantation group and the blank control group. The rats were sacrificed separately 14 days after transplantation by inhalation of increased concentrations of carbon dioxide.
Generation and culturing of ENSCs
ENSCs were isolated and cultured from the embryonic bowel of rats in vitro, then seeded into the Corning flasks coated with HFN and were kept in the culture in a humidified incubator at 37°C, 5% CO2 concentration. Half of the volume was replaced with fresh medium once in 2 days [13].
Identification of the ENSCs
- Morphological identification: We observed when the neurospheres could appear and investigated whether there are some connections among the cells or not.
- Immunohistochemistry identification: All cells were fixed in 4% (w/v) paraformaldehyde for 15 minutes at RT and then were rinsed extensively with PBS. Then the neurospheres were blocked with the blocking solution (PBS containing 1% BSA, 0.5% normal goat serum and 0.3% Triton X-100). The primary antibodies diluted in PBS were applied overnight at 4°C. Negative controls lacking the primary antibody were included in each series, and other experimental procedures were based on the instructions of the reagents. Nuclei were counterstained with Hoechst33258.
PKH-26-labeled ENSCs
ENSCs were digested in 0.25% Trypsin-EDTA solution. Single-cell suspension was obtained, then the suspension was filtered through a 400-mesh filter, centrifuged and washed with DMEM/F12. After washing, the suspension was centrifuged at 1200r/min for 5 minutes. After removal of the supernatant, 0.5ml diluent C was added. Cells were resuspended to be fully dispersed. Before staining, 4×10-6mol/L PKH-26 staining solution was placed in a centrifuge tube. 0.5ml 2X cells were added in 0.5ml 2X dye, and rapidly stirred with a pipette for 2-5 minutes incubation at 25°C. Serum was added to terminate the staining reaction. The above reaction solution was diluted with an equal volume of complete medium, centrifuged, washed three times with D-Hank’s solution. After centrifugation, the supernatant was discarded, and the cell masses were resuspended to obtain suitable concentration. We evaluated the staining efficiency of fluorescence and fluorescence intensity with a fluorescence microscope (absorption wavelength 551/488nm, excitation wavelength 567nm).
Transplantation
The PKH-26-labeled ENSCs before transplantation were prepared, and a micro syringe was used to draw 1ml suspension. Rats were fasted for 12 hours before operation, and then were anesthetized by injection 10% chloral hydrate (3mL/Kg) intraperitoneally, and then were set on the operating table. Abdominal shaving, 5% iodophor sterilize, we choose the straight abdominal incision about 2cm long, pulled out from the stomach, and exposed the pylorus. After marking with the suture line, we injected the 0.1mL suspension into the muscular layer of pylorus. Control group were injected with the same volume of saline. The wound was sutured by layer, and the rats were marked with number. Daily activities of the rats were recorded.
Frozen sections observation and H&E counterstaining
Pylorus tissues were obtained from the rats. The mesenteries of the tissues sample were completely removed. The remaining luminal content was rinsed by saline, the tissues were then embedded with OCT and made into serial sections with a 9 μm thickness. Images were captured under light and fluorescence microscopy, sections were then counterstained with H&E, images were recorded and reconstructed by software.
Immunohistochemistry and image reconstruction
Sections of the pylorus were fixed for 20 min in 4% paraformaldehyde, washed in 0.1% BSA contained PBS buffer, blocked with solution (PBS containing 0.1% Triton X-100, 10% normal goat serum and 1% BSA) for 45min. The primary antibodies were added and incubated over night at 4°C. Sections were washed 3 times with PBS buffer and incubated with secondary antibodies for 1 hour at room temperature. After the reaction, sections were covered with anti-fluorescence quenching agent. Nuclear staining with Hoechst33258 (50μg/ml) was performed, and sections were rinsed with PBS buffer. Images were captured and reconstructed by software.
Figures
Results
Morphology dynamic observation of primary cell culture:
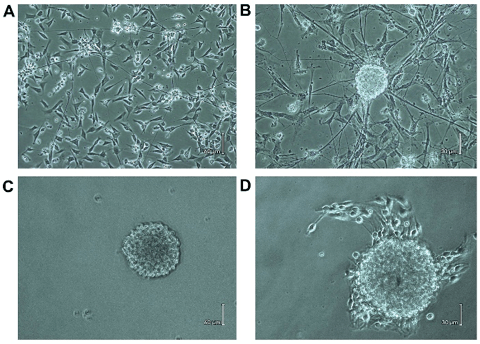
Different forms of adherent cells and some cell debris were visible on day 1 of culture. Cell colony was formed, and part of the region of cell clumps with long neurites could be observed on day 2. After 3-5 days of culture, cell density was reduced obviously because of the death of non-neurons; the adherent cell clusters were grown bigger and some live cells were migrated to other places, in which long axons appeared between these cells (Figure. 1A). The NLBs appeared on the top of the adhered cells after 6-7 days (Figure. 1B). Free-floating NLBs were formed after 9-10 days culture (Figure. 1C), also some cells were adhered to the culture dish and occasionally produced neurite-like processes. A few days later, some ENSCs migrated from NLBs (Figure. 1D), and the secondary nerve plexus appeared.
Immunocytochemistry characterization of ENSCs
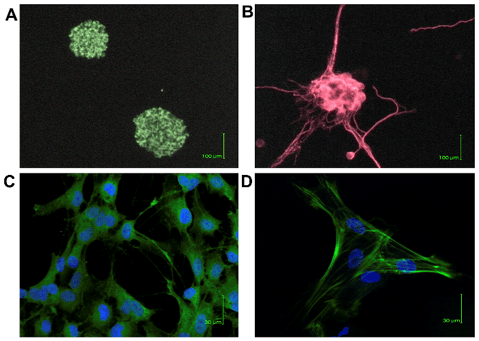
ENSCs formed characteristic neurospheres of approximately 20 μm in diameter within 2 weeks in culture, which comprised cells expressing stem cells markers such as Nestin (Figure. 2A). After differentiation, some cells expressed ENS marker as TUJ-1(Figure. 2B); and other cells expressed glial marker GFAP (Figure. 2C) and smooth muscle marker SMA (Figure. 2D), which showed the ENSCs had the potential to be glial or smooth muscle cells.
Survival of ENSCs after pylorus muscular layer transplantation
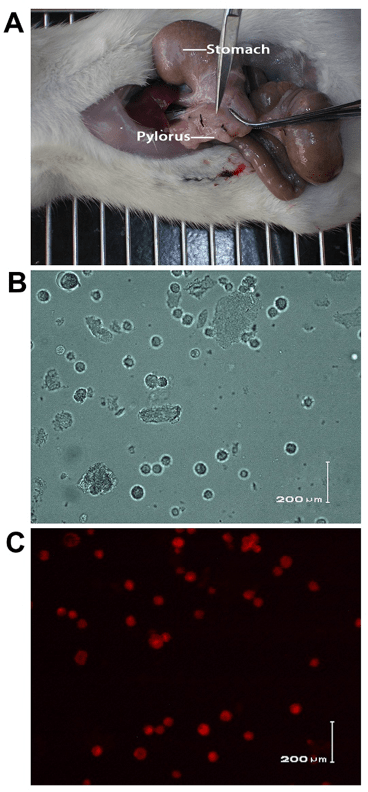
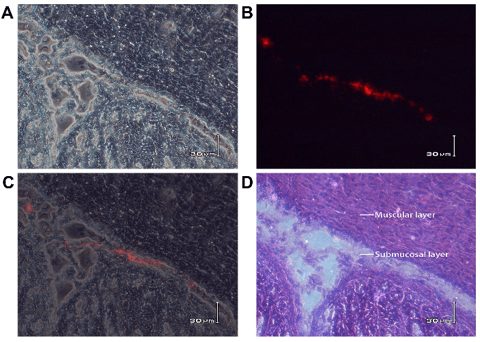
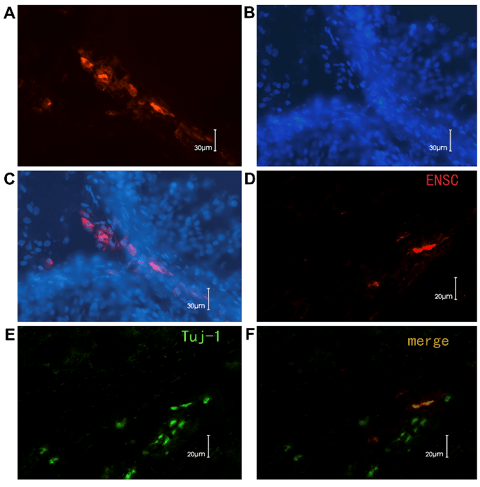
The muscular layer of pylorus is thick enough to inject the ENSCs and is easy to observe after transplantation (Figure. 3A). The fluorescent dye, PKH26, which mainly binds to the cell membrane, has been used as the cell tracer to locate the transplanted cells in the host for a long time. Figures 3B and Figures 3C showed ENSCs under white light and fluorescent light after labeling. The PKH26-labeled ENSCs were in a circular form after being labeled, displayed red fluorescence and were with smooth edge under fluorescent microscopy, which showed a good bioactivity of the labeled cells. The red fluorescent cells could be observed at different times after transplantation. The distribution of the transplanted cells became regular and migrated from the muscular layer to the submucosal layer (8/10) (Figure. 4). 2 weeks later,the PKH-26 labeled ENSCs could be observed with the Hoechst33258 nuclear staining and the normal cellular morphology was observed, where the cells were alive and revealed that ENSCs could survive for a long time in the digestive tract (Figure.5A, B, C). Some grafted cells expressed Tuj-1, a specific marker of neurons, which suggested the differentiation towards neurons (Figure. 5D, E, and F).
Discussion
An irregular colonization where differentiation was inappropriate, or the yield of migrating cells was insufficient which led to the inevitably serve motility disorders in humans, such as Hirschsprung's disease. Here, the distal part of the ENS was neglected, leading to a segment of gut with varying length that is either not fully innervated or completely aganglionic [14].
In this study, we cultured ENSCs, tracer labeled technology to explore the way of ENSCs transplantation for the treatment of intestinal ganglion cell deficiency, which acts as a potential therapeutic method for Hirschsprung’s disease. The colon wall tissues of the infant rats were thin with no conducive to the injection mode of cell transplantation, but the pyloric wall had clear structure, muscle layer thickness which facilitated the transplantation injection and the observation of cell migration. By morphological and immunological identification, the transplanted stem cells were differentiated into enteric neurons, glial cells, smooth muscle cells and so on. These results were similar to the report of Almond [10]. who had transplanted intestinal neurospheres into the fetal rat intestinal tissue. In our study, the recipient animals were SD rats at age 3-4 weeks, which were similar to the human infants, the choice of experimental animal age provided a certain reference value.
The pylorus area of the infant rats are clear in the anatomic structure, also can be an easy target of transplantation injection. We observed that the transplanted cells maintained a normal cell morphology as the time prolonged, the distribution of the transplanted cells were irregular at the beginning and were turned into regular with the time extension and had a tendency to migrate to the submucosal area. Is this area good for the growth of ENSCs or graft versus host reation? We could not tell this as it still needed further research. But at least 2 weeks later, the red PKH26 labeled cells were still visible, the cell morphology was natural, and the nuclear Hoechst33258 staining was clear, suggesting that the ENSCs could survive well after transplantation.
In this study, we reconstructed the images after transplantation, found that transplanted ENSCs and the host enteric neural cells were mixed with each other, and the transplanted cells expressed Tuj-1, which commonly regarded as early marker of neuronal differentiation, considered to be one of the earliest signs of stem cell differentiation into neurons [15]. The expression of Tuj-1 of transplanted neural stem cells in pylorus showed that these cells were differentiated to mature neurons in vivo.
After 14 days of ENSCs transplantation, red PKH26 labled cells were still scattered in the pylorus wall sections, it revealed that ENSCs had a good survival, migration and differentiation in the host microenviroment. Our results provided the experimental basis for further study of ENSC transplantation in the treatment of intestinal neuronal development defect. It was the first step to identify whether ENSCs transplantation can be applied to cure congenital or acquired intestinal neuromuscular diseases. Whether the transplanted cells have the corresponding function or cannot be detected at present. But to our knowledge, some report showed that the brain stem cells transplanted in the colon of aganglionic mice and the regulation of the intestinal nerve partly recovered, which provided us some hints [16].
In vitro intestinal organ culture experiments showed that, in the early embryo period, intestinal neuronal developmental defects could be cured by transplantation of neural stem cells [17]. The research of ENSCs transplantion therapy for intestinal neuromuscular disorders is still in the initial stage, and there were many problems and dilemmas associated with the investigation such as (I) whether the biological features of ENSCs after transplantation in vivo were similar as the features of in vitro culture? (II) How to detect the function of ENSCs [18]?
In conclusion, our study showed that transplanted ENSCs can survive and migrate into the pylorus wall of rats. Adequate isolation and expansion technologies combined with the positive perspectives of the appropriate microenvironments gave rise to the hope that the transplantation of ENSCs showed therapeutic potential for HD which can be a realistic goal in the near future.
Acknowledgements
The work was financially support by the Zhejiang Natural Science Foundation (No.: LY13H040011), project grant funding from Health and Family Planning Commission of Zhejiang Prpvince(NO.2018KY128) ,Project grant funding of Wenzhou Science and Technology Bureau (NO.Y20160098)).
Conflict of Interest
The authors declare that they have no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received 14 May, 2018Accepted 24 May, 2018
Published 31 May, 2018
Copyright
© 2018 Zhongrong Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository. All rights reserved.Author Info
Corresponding author
Zhongrong LiDepartment of Pediatric Surgery, The 2nd Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
Figures & Tables





References
1. Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286-294. [Crossref]
2. Grundmann D, Klotz M, Rabe H, Glanemann M, Schafer KH (2015) Isolation of high-purity myenteric plexus from adult human and mouse gastrointestinal tract. Sci Rep 5: 9226.
3. Obermayr F, Hotta R, Enomoto H, Young HM (2013) Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol 10: 43-57. [Crossref]
4. Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO (2003) GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130: 2187-2198. [Crossref]
5. Amiel J, Lyonnet S (2001) Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet 38: 729-739. [Crossref]
6. Heanue TA, Pachnis V (2007) Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci 8: 466-479. [Crossref]
7. Burns AJ, Thapar N (2014) Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol 11: 317-328. [Crossref]
8. Hetz S, Acikgoez A, Voss U, Nieber K, Holland H, et al. (2014) In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One 9: 93605. [Crossref]
9. Rauch U, Hansgen A, Hagl C, Holland-Cunz S, Schafer KH (2006) Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis 21: 554-559. [Crossref]
10. Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH (2007) Characterisation and transplantation of enteric nervous system progenitor cells. Gut 56: 489-496. [Crossref]
11. Metzger M, Bareiss PM, Danker T, Wagner S, Hennenlotter J, et al. (2009) Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology 137: 2063-2073. [Crossref]
12. Hagl CI, Heumuller-Klug S, Wink E, Wessel L, Schafer KH (2013) The human gastrointestinal tract, a potential autologous neural stem cell source. PLoS One 8: 72948. [Crossref]
13. Zhu LB, Liu ZJ, Wang AH, Li ZR, Chen XM (2007) A report on the culture of enteric neural stem cells in rats. Chinese J Experimental Surgery 24: 122.
14. Hagl CI, Rauch U, Klotz M, Heumuller S, Grundmann D, et al. (2012) The microenvironment in the Hirschsprung's disease gut supports myenteric plexus growth. Int J Colorectal Dis 27: 817-829. [Crossref]
15. Jacobs JS, Miller MW (2000) Cell cycle kinetics and immunohistochemical characterization of dissociated fetal neocortical cultures: evidence that differentiated neurons have mitotic capacity. Brain Res Dev Brain Res 122: 67-80. [Crossref]
16. Shu Xg, Chen jb, Wang Zy, Wang Gb (2009) The neural stem cells transplantation in the treatment of aganglionic mice. Chinese J Experimental Surgery 26: 755-757.
17. Burns AJ, Pasricha PJ, Young HM (2004) Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil 1: 3-7. [Crossref]
18. Shu X, Meng Q, Jin H, Chen J, Xiao Y, et al. (2013) Treatment of aganglionic megacolon mice via neural stem cell transplantation. Mol Neurobiol 48: 429-437. [Crossref]
